Abstract
Yolk sac tumors are rare malignant germ cell neoplasms that usually arise from the gonads. Extragonadal yolk sac tumors (EGYSTs) frequently occur in the mediastinum in post-pubertal females. EGYSTs in the pelvis are extremely rare, and to date, only thirteen cases have been reported in the English literature. Among them, the primary EGYST of the pelvic peritoneum in post-pubertal females has only been reported in ten cases. The present case describes a 26-year-old female diagnosed with primary peritoneal yolk sac tumor located in the rectouterine pouch. We report clinical and tumor imaging features, including ultrasound, computed tomography (CT), magnetic resonance images (MRI), positron emission tomography-computed tomography (PET-CT), and present a review of the literature.
In female patients, yolk sac tumors are encountered mostly in the ovaries, and only 10% of the tumors arise in extragonadal sites. Extragonadal germ cell tumors arise in various sites, such as the sacrococcygeal region, mediastinum, vagina, vulva, liver, central nervous system, pineal gland, stomach, omentum, pelvis and other sites (1). In post-pubertal females, the most common location of the extragonadal yolk sac tumor (EGYST) is the mediastinum. The primary EGYST of the pelvis is extremely rare in this age group. A few reports have described the radiological and pathological features of the primary pelvic peritoneal yolk sac tumors in post-pubertal females (23456789). Herein, we report a case of a 26-year-old female through the use of ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT), as well as provide a review of the literature.
A 26-year-old nulliparous woman visited an outpatient clinic complaining of dysuria for the previous two months. She menstruated regularly at intervals of thirty days and complained of dysmenorrhea. She had no previous medical history. On her transvaginal ultrasound, a 9.5 × 6.1 cm-sized, well-defined, solid mass with a small central anechoic component, was detected in the pelvic cavity (Fig. 1). A contrast-enhanced abdomen and pelvis CT scan revealed a 9.3 × 7.0 cm-sized predominantly solid mass with heterogeneous enhancement in the rectouterine pouch. The mass closely abutted the posterior aspects of the uterus and right adnexa (Fig. 2). A dynamic contrast-enhanced pelvic MRI showed a predominantly solid mass with T2 intermediate-to-high signal intensity and internal high signal intensity components on the T1-weighted image. The central T1 high signal intensity component showed diffusion restriction, suggesting a foci of hemorrhage (Fig. 3). Avid uptake of the pelvic mass was noted on the whole-body 18F-FDG (18F-fluorodeoxyglucose) PET/CT scan, and the standardized uptake value (SUV) max was 6.2 (Fig. 4). No other abnormal FDG uptake suggesting metastasis was noted. The serum alpha-fetoprotein (AFP) level was markedly elevated at 7500 ng/mL. The serum cancer antigen 125 (CA 125), CA 19-9 and β-hCG values were in the normal range (26.60 U/mL, 8.13 U/mL, <1.20 mIU/mL, respectively). Based on radiological findings and laboratory test results, the patient was initially diagnosed with malignant germ cell tumor of the right ovary.
For the diagnosis, explorative laparotomy was performed. About a 10 cm-sized mass was detected in the uterine cul-de-sac with adhesion to the rectum. The mass was independently located in the cul-de-sac, and there was no gross abnormality in the uterus or both adnexa. Microscopically, a tumor mass shows various histologic growth patterns, including solid, reticular/microcystic, tubular and papillary growth. Occasionally, it contains tubulopapillary sinusoidal structures with a central vascular core and cuboidal to columnar epithelial-like cell lining (Schiller-Duval sinuses or bodies), diagnostic of the yolk sac tumor (Fig. 5a). Tumor cells consist of polygonal or columnar cells with abundant, clear or eosinophilic cytoplasm and have ovoid vesicular nuclei and inconspicuous nucleoli. Immunohistochemically, these cells are positive for AFP (Fig. 5b). Seventeen days after the initial operation, a following secondary staging surgery was performed: laparoscopic right salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, omentectomy and appendectomy. On the final pathologic report, there was a residual yolk sac tumor in the resected pelvic peritoneum. However, there was no evidence of the tumor in the right ovary, lymph nodes and omentum. A follow-up AFP level obtained one day after the second operation decreased to 4996.8 ng/mL. The patient received adjuvant chemotherapy with a BEP (bleomycin, etoposide and cisplatin) regimen. To date, two months after the diagnosis of the primary pelvic peritoneal EGYST, the patient has received one cycle of adjuvant chemotherapy, and she has no evidence of the disease.
Primary germ cell tumors of extragonadal origin are rare. The estimated incidence is reported to be 3–5% of all the adult malignant germ cell tumors (10). The pathogenesis of extragonadal yolk sac tumor (EGYST) is not well identified. One theory of its origin explains that it arises from transformation of primordial germ cells arrested in various locations along their paths of embryonic migration On the other hand. another suggests that it arises from misplaced primordial germ cells with aberrant migrations (7). EGYST can be divided into broad categories according to age groups. The pathologic features, prognosis, and location of the tumor are different among the various age groups (11). Congenital or neonatal tumors are frequently located along the midline, most commonly in the sacrococcygeal region, and are usually admixed with teratoma. In the pre-pubertal age, the location of the EGYST is similar to the neonates. However, they are commonly pure yolk sac tumor (YST). EGYST frequently occurs in vaginas of young females under the age of 3 years. In the post-pubertal age, the mediastinum is the most common location, and the EGYST is usually admixed with other germ cell tumors (11). The primary EGYSTs of the pelvis in post-pubertal females are extremely rare. Thus, EGYST might not be readily considered in differential diagnosis of tumors arising in this location, as in our case. In addition, YST and somatic carcinoma may become pitfalls in pathologic diagnosis because they share some overlapping histopathological and immunohistochemical features with EGYST.
There have been thirteen reported cases of EGYST of the pelvis, and only ten occurred in post-pubertal females (23456789) (Table 1). The mean age of the reported patients was 27.5 years (range, 17 to 36 years). Two patients were pregnant at the time of diagnosis. Abdominal pain and palpable mass were the prevailing symptoms. Serum AFP is a useful marker for the diagnosis of yolk sac tumors. The serum AFP from the reported cases ranged from 1136 ng/mL to 441,611 ng/mL. In our case, the serum AFP was elevated to 7500 ng/mL, which played an important role in the diagnosis of yolk sac tumor. The tumor size described in the reported cases ranged from 6 cm to 15.4 cm (mean, 9.1 cm). Majority of the cases showed advanced diseases at the time of diagnosis. Extrapelvic metastases in the peritoneum, omentum and liver were reported in six patients.
The reported imaging findings of the ovarian yolk sac tumors include a unilateral, enhancing, mixed solid and cystic mass with extensive necrosis and hemorrhage. The cystic components are diffusely scattered throughout the mass, and the sizes vary from a few millimeters to 2 cm in diameter (12). Since the extragonadal germ cell tumors morphologically resemble their gonadal counterparts, the primary peritoneal yolk sac tumors are presumed to show similar characteristics on the imaging studies (13). The imaging findings of EGYST of the pelvis in post-pubertal females were described in only five cases (56789). Majority of the cases showed heterogeneous solid or semi-solid masses with hemorrhage or necrosis. One case appeared as a relatively homogeneous hyperechoic mass on US, and was a well-defined soft tissue mass mimicking a subserosal uterine myoma (5). In the present case, the mass was seen as a predominantly solid mass with heterogeneous echogenicity on ultrasound and heterogeneous enhancement on contrast-enhanced CT and MRI. There were small foci of hemorrhage within the mass. No previous report has described the preoperative 18F-FDG PET/CT scan findings of the primary EGYST in the pelvis. Only one report showed utility of 18F-FDG PET/CT scan in treatment monitoring after right salpingo-oophorectomy with cytoreductive surgery and adjuvant chemotherapy with BEP regimen in advanced disease (8). In that case, serial follow-up 18F-FDG PET/CT scans demonstrated a gradual decrease in FDG accumulation within the residual tumor (SUVmax 5.7 in 2nd cycle; SUVmax 3.2 in 4th cycle; SUVmax 0.8 in 6th cycle). In this report, we described the first 18F-FDG PET/CT scan findings of primary EGYST in the pelvis at the time of the initial diagnosis. Marked FDG accumulation was noted within the mass, and the SUVmax was 6.2, which is a value similar to that in a reported case of immature teratoma of the ovary (SUVmax, 5.8) and cases of epithelial origin malignant ovarian tumor (mean SUVmax, 7.9) (1415).
Since the EGYST of the pelvic peritoneal region is extremely rare and occurs in relatively young child-bearing women (mean age, 27.5 years), appropriate diagnosis and treatment is important in preserving the ovarian function and childbearing capability. Among the reported EGYST in the pelvic peritoneal region of post-pubertal females, explorative laparotomy with mass removal and other procedures were performed, except for one inoperable case. In three cases, the uterus and both ovaries were preserved. All patients received adjuvant chemotherapy with the exception of one, who refused treatment. The regimens included VAC (vincristine, actinomycin-D and cyclophosphamide), BEP (bleomycin, etoposide and cisplatin), EP (etoposide and cisplatin) and BP (bleomycin and cisplatin). Although the majority of the patients were at the advanced stage at the time of diagnosis, the outcome was not poor. The follow-up periods ranged from 0 to 8.5 years, and 70% (7/10) of the patients were alive with no evidence of disease.
In conclusion, primary EGYST of the pelvis in postpubertal females is a rare disease. Herein, we report a rare case of a primary pelvic peritoneal yolk sac tumor in a 26-year-old female who was initially treated with cul-de-sac mass removal followed by laparoscopic right salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, omentectomy and appendectomy, followed by adjuvant chemotherapy. We described detailed imaging features from multiple modalities of this rare disease with an associated literature review. Since primary EGYST of the pelvis occurs in women of reproductive age, an appropriate diagnosis and management based on the imaging features and laboratory results are important for patient outcomes.
Figures and Tables
Fig. 1
On transvaginal ultrasound, a 9.5 × 6.1 cm-sized, well-defined, solid mass with heterogeneous echogenicity and a small central anechoic component (white arrow) was seen in the pelvic cavity.
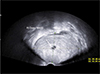
Fig. 2
Axial contrast-enhanced computed tomography scan revealed a 9.3 × 7.0 cm-sized predominantly solid mass in the rectouterine pouch with heterogeneous enhancement. The mass closely abutted the posterior aspect of the uterus (white solid arrow) and right adnexa (dashed white arrow).
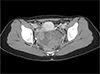
Fig. 3
Dynamic contrast-enhanced pelvic magnetic resonance images showing the mass located in the cul-de-sac that appeared predominantly solid with intermediate-to-high signal intensity on a sagittal T2-weighted image (a). Note that both normal ovaries are observed as separate from the mass on a coronal T2-weighted image (b, white arrows). There is a central focal area of high signal intensity on both T1- and T2-weighted images with diffusion restriction (white arrowheads in a, c, d), suggesting hemorrhage.
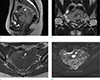
Fig. 4
Avid uptake of the pelvic mass was noted on the 18F-fluorodeoxyglucose positron emission tomography-computed tomography scan (maximum standardized uptake value, SUVmax: 6.2).
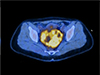
Fig. 5
(a) On the histological examination, the mass was predominantly composed of a loose network of anastomosing channels or micro cysts consisting of tumor cells with varying amounts of clear or eosinophilic cytoplasm. Many Schiller-Duval bodies (black arrows) were present (Hematoxylin and Eosin staining, × 100). (b) The tumor cells were positive for alpha-fetoprotein (AFP, × 100).

Table 1
Image Findings, Serum AFP Level and Treatment of Primary Yolk Sac Tumor in the Pelvis of Postpubertal Females

BEP = bleomycin, etoposide and cisplatin; BP = bleomycin and cisplatin; BSO = bilateral salpingo-oophorectomy; DOD = died of disease; EP = etoposide and cisplatin; ND = not described; NED = no evidence of disease; P&PA-LND = pelvic and para-aortic lymph node dissection; TAH = total abdominal hysterectomy; VAC = vincristine, adriamycin-D (dactinomycin), cyclophosphamide
References
1. Gilbert KL, Bergman S, Dodd LG, Volmar KE, Creager AJ. Cytomorphology of yolk sac tumor of the liver in fine-needle aspiration: a pediatric case. Diagn Cytopathol. 2006; 34:421–423.
2. Huntington RW Jr, Bullock WK. Yolk sac tumors of extragonadal origin. Cancer. 1970; 25:1368–1376.
3. Thiele J, Castro S, Lee KD. Extragonadal endodermal sinus tumor (yolk sac tumor) of the pelvis. Cancer. 1971; 27:391–396.
4. Clement PB, Young RH, Scully RE. Extraovarian pelvic yolk sac tumors. Cancer. 1988; 62:620–626.
5. Dede M, Pabuccu R, Yagci G, Yenen MC, Goktolga U, Gunhan O. Extragonadal yolk sac tumor in pelvic localization. A case report and literature review. Gynecol Oncol. 2004; 92:989–999.
6. Tseng MJ, Jung SM. Primary extra-gonadal yolk-sac tumour of pelvis with unusual presentation. Lancet Oncol. 2007; 8:179–180.
7. Pasternack T, Shaco-Levy R, Wiznitzer A, Piura B. Extraovarian pelvic yolk sac tumor: case report and review of published work. J Obstet Gynaecol Res. 2008; 34:739–744.
8. Baba T, Su S, Umeoka S, et al. Advanced extragonadal yolk sac tumor serially followed up with (18)F-fluorodexyglucose-positoron emission tomography and computerized tomography and serum alpha-fetoprotein. J Obstet Gynaecol Res. 2012; 38:605–609.
9. Rudaitis V, Mickys U, Katinaite J, Dulko J. Successful treatment of advanced stage yolk sac tumour of extragonadal origin: a case report and review of literature. Acta Med Litu. 2016; 23:110–116.
10. Garnick MB, Canellos GP, Richie JP. Treatment and surgical staging of testicular and primary extragonadal germ cell cancer. JAMA. 1983; 250:1733–1741.
11. McKenney JK, Heerema-McKenney A, Rouse RV. Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv Anat Pathol. 2007; 14:69–92.
12. Shaaban AM, Rezvani M, Elsayes KM, et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics. 2014; 34:777–801.
13. Ravishankar S, Malpica A, Ramalingam P, Euscher ED. Yolk sac tumor in extragonadal pelvic sites: still a diagnostic challenge. Am J Surg Pathol. 2017; 41:1–11.
14. Wang M, Jiang S, Zhang Y, et al. The application of 18F-FDG PET/CT in ovarian immature teratomas when pathological examination results contradict clinical observations: a case report. Medicine (Baltimore). 2017; 96:e9171.
15. Takehara K, Konishi H, Kojima A, et al. SUV max of FDG-PET/CT as a prognostic factor in ovarian clear cell adenocarcinoma. J Clin Oncol. 2014; 32:e16509.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download