Abstract
Perirectal cystic lesions are rare entities, for which only a relatively few research publications are available. These lesions are heterogeneous in nature and can range from benign lesions to malignant lesions; thus, they are sometimes difficult to differentiate. Some studies have reported on retrorectal or presacral cystic lesions, but to our knowledge, there have been only a few reports on perirectal cystic lesions. Cystic lesions arise from the retrorectal space as well as the rectal lumen or adjacent organ, and they should be differentiated based on their characteristics and anatomic location. Thus, we comprehensively studied diseases with a cystic component around the rectum, which are perirectal cystic lesions. A clinical challenge with perirectal cystic lesions is that it is sometimes difficult to distinguish malignant lesions from benign lesions and is thus difficult to determine the extent for surgical excision. We thus attempted to identify benign and malignant imaging features of perirectal cystic lesions.
Some studies have reported on retrorectal or presacral cystic lesions, but to our knowledge, there have been only a few reports on perirectal cystic lesions. Cystic lesions arise from the retrorectal space as well as the rectal lumen or adjacent organ, and they should be differentiated based on their characteristics and anatomic location. Thus, we comprehensively studied perirectal cystic lesions, which contain cystic a component around the rectum.
Most perirectal cystic lesions are incidentally discovered, and patients can present with a variety of symptoms, including abdominal pain, bowel obstruction, perforation, intussusception, and intestinal bleeding (1). Because of the non-specificity of the associated symptoms, perirectal cystic lesions are frequently overlooked.
Diagnosis of perirectal cystic lesions had been difficult because of the non-specific radiologic features and symptoms. However, computed tomography (CT) and magnetic resonance imaging (MRI) allow evaluation of the entire intestinal wall layer and the perirectal tissue, thus facilitating further characterize these lesions (2). In particular, as MRI can accurately show the perirectal anatomy, it has a high radiologic diagnostic value (2). Thus, MRI has primarily been used to study perirectal cystic lesions.
Perirectal cystic lesions are heterogeneous in nature and range from benign lesions to malignant lesions, which are sometimes difficult to distinguish. The clinical issue is if a perirectal cystic lesion should be considered malignant, what imaging features indicate malignancy, and thus to what extent should surgical excision be performed (3). In this study, we differentiate the imaging features of two categories of perirectal cystic lesions, malignant and non-malignant lesions, and identify the image characteristics of each category by showing and discussing the CT and MRI findings.
During embryogenesis, the tailgut is the most caudal part of the hindgut distal to the future anus. It normally involutes by the eighth week of embryonic development. If a tailgut remnant persists, it may become a tailgut cyst (3). It is usually a discrete and well-defined mass of variable attenuation, as seen on CT depending on its contents (Fig. 1). A calcified cystic wall may be observed. On MRI, a tailgut cyst is usually multilocular and has low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Fig. 2). However, it may have high signal intensity on T1-weighted images due to the presence of mucinous materials, high protein content, or hemorrhage in the cyst (3).
The majority of perirectal abscesses are caused by the spread of disease from adjacent structures, with an anorectal inflammatory condition the most common cause of abscess.
Rectal perforations, iatrogenic causes, adjacent cutaneous infection, and trauma are additional causes of abscess.
Abscesses are fluid collections, shown as well-defined areas of low attenuation (<18 Hounsfield units) (45). In the enhanced image, ring enhancement can be observed on CT and MRI. Secondary findings include obliteration of adjacent tissue planes containing gas bubbles and air-fluid levels (4) (Fig. 3).
Epidermoid cyst is a benign congenital lesion of ectodermal origin. It develops from an ectodermal tissue remnant that is mislaid during embryogenesis due to defects in the development of adjacent structures (8). An epidermoid cyst can be observed throughout the body,
but is rare in the perirectal region (9). On CT images, an
epidermoid cyst generally appears as thin-walled, cystic
masse with fluid density, and it may contain calcification
(10). it may show high attenuation on precontrast CT scan,
possibly due to a high protein content, previous bleeding, or
deposition of iron-containing pigments (10).
A dermoid cyst results from the abnormal closure of the
ectodermal tube in the embryogenesis and is lined with
stratified squamous epithelium. A dermoid cyst contains
skin appendages and may, thus, include variable amounts
of the fatty component (10) (Fig. 6). On CT images it is
generally round and well-circumscribed, with a thin outer
wall. MRI can be used to enable detection of the fat
component of the dermoid cyst demonstrating increased signal intensity on T1-weighted images and decreased
signal intensity with fat-suppression techniques (12).
Intramural hematoma of the gastrointestinal tract is an uncommon and mostly caused by blunt trauma (13). However, 15-36% of intestinal intramural hematomas are spontaneous, and unrelated to hematologic disorders or anticoagulant use (13).
Most intestinal intramural hematoma patients present with obstructive signs (13), yet some of these patients possibly have abdominal symptoms due to rupture of the hematoma into the abdominal cavity (13).
CT scan is accurate for detecting gastrointestinal wall hematomas. On CT images, intramural hematomas are delineated as well-defined, hyperdense homogeneous masses (14). Unlike other gastrointestinal neoplasms, hematomas usually do not have calcification and do not infiltrate other organs (14) (Fig. 7).
Diffuse cavernous hemangioma is a rare, benign vascular abnormality consisting of an extended network of vascular channels involving the entire enteric wall, which can infiltrate the adjacent connective tissue. A history of chronic rectal bleeding in a young patient can be a clinical clue.
On colonoscopy, diffuse cavernous hemangiomas are usually soft, submucosal lesions that are purple and collapse on insufflation.
On CT images, these lesions are observed as circumferential, with enhanced wall thickening of the involved rectal segment as well as vascular engorgement within the nearby mesorectum. Multifocal, calcified, intralesional phleboliths may be the most integral sign for the identification of rectal hemangioma (Fig. 8).
On MRI, diffuse cavernous hemangioma shows high signal intensity on T2-weighted images in a markedly thickened rectal wall, considered to be caused by the slow flow of the vascular network. Additionally, the perirectal fat may demonstrate heterogeneous signal intensity on T2-weighted images due to the small, twisted feeding vessels.
Colitis cystica profunda is an uncommon benign condition characterized by mucin-filled cysts in the submucosa and is frequently associated with the solitary ulcer and rectal prolapse syndromes (15). The diagnosis of this entity is crucial as it can mimic rectal cancer and, may thus result in unnecessary surgical resection.
On CT images, the lesions have been described as non-infiltrating submucosal masses with loss of perirectal layers of the fatty tissue and thickening of the levator ani muscle (16) (Fig. 9). MRI findings demonstrated submucosal hyperintense nodules on T2-weighted images with no remarkable contrast enhancement (16).
Primary extracranial meningioma is a rare disease, occurring mostly in the head and neck. The reported incidence is less than 2% of all meningiomas (17). A primary extracranial meningioma occurrence in the perirectal area is even rarer and its pathogenesis is unclear (18).
The radiologic findings of primary extracranial meningioma are known to be similar to intracranial meningioma.
On CT images a primary extracranial meningioma demonstrates lobulated contour, heterogeneous enhancement with or without internal calcification (19) (Fig. 10). It can show central areas of a cystic/necrotic portion. The vascularity in the pelvis is lesser than the vascularity of the brain, so primary extracranial meningioma in the perirectal area is less perfused and more necrotic (18).
Rectal mucinous adenocarcinoma is a histologic subtype that represents 5-15% of all rectal cancers (20). Mucinous adenocarcinoma is characterized by an abundance of extracellular mucin that exceeds 50% of the tumor stroma as determined with histopathologic examination (21). They are known to be associated with benign inflammatory conditions such as perianal abscesses, and Crohn's disease (22).
CT findings suggesting a mucinous adenocarcinoma include a multilocular cystic mass with peripheral calcification (23). MRI features indicating a mucinous adenocarcinoma include masses filled with markedly hyperintense content, as seen on T2-weighted images, enhancing solid components, mesh-like internal enhancement, contrast enhancement of peripheral structures or peritumoral areas, and regional areas of lymph node enlargement (23) (Fig. 11).
Malignant transformation of the epithelial component of a tailgut cyst has only been reported on rare occasions (24). Malignancies that have been reported within a tailgut cyst include adenocarcinomas, carcinoid tumors, neuroendocrine carcinomas, endometrioid carcinoma, adenosquamous carcinoma, squamous cell carcinoma, and sarcoma (2526). If malignant transformation occurs, CT may reveal the loss of discrete margins and involvement of contiguous structures (27) (Fig. 12). Additionally, a malignant change within a cyst may be observed as an irregular wall thickening or a polypoid mass with intermediate signal intensity, as observed on T1- and T2-weighted images with enhancement after the IV administration of paramagnetic contrast material (2829).
A mucinous adenocarcinoma associated with a chronic fistula-in-ano is rare, and the diagnosis is often difficult (30). The absence of a tumor within the intestinal lumen and the slow growth of a lesion hidden within the ischioanal fossa and perineum make early diagnosis difficult (31).
MRI features of a fistula-in-ano include masses filled with markedly hyperintense content on T2-weighted MR images, enhancing solid portion, mesh-like enhancement pattern, a fistula between the lesion and the anus, enhancement of peritumoral areas, and regional areas of lymph node enlargement (32).
A primary retroperitoneal teratoma with malignant transformation is extremely rare in adults, and that of extragonadal origin is even rarer and has been reported in the anterior mediastinum, stomach, brain, retroperitoneum, and sacrococcygeal region (3334). As teratomas with malignant transformation are usually chemoresistant and recurrence is common, complete surgical resection of the residual or recurrent disease thus appears to offer the best path to prolonged patient survival (33).
On CT and MRI, benign and malignant teratomas cannot be consistently distinguished according to size or the presence of a solid mass. Suggestive findings of the presence of malignant transformation are irregular wall thickening of the cystic area and extension into the adjacent structures, as demonstrated on CT and MRI (33)
(Fig. 14).
Gastrointestinal stromal tumors (GISTs) arise from the interstitial cells of Cajal and are the most common nonepithelial tumors of the gastrointestinal tract. GISTs occur most commonly in the stomach (60-70%) followed by the small intestine (20-25%); however, GISTs in the rectum are extremely rare (5%). It was reported that GISTs account for 0.6% of all malignant rectal tumors (35).
The CT features of GISTs vary markedly, depending on the size and aggressiveness of the tumor and the time of presentation during the course of the disease. GISTs are typically large, hypervascular, enhancing masses and are often heterogeneous due to necrosis, hemorrhage, or cystic degeneration at the time of their presentation (36). Rectal GISTs generally manifest as large, eccentric masses growing beyond the rectal wall (34).
A perirectal cystic lesion may be a diagnostic challenge because of its non-specific symptoms and radiologic findings. The presence of a solid component in a cystic lesion and invasion into adjacent structures are key imaging findings of malignancies that distinguish them from benign lesions, except for teratoma and primary extracranial meningioma. Teratoma and extracranial meningioma are exceptional as solid components cannot be used to distinguish benign and malignant.
Key imaging findings for perirectal cystic lesions and comparison of benign and malignant lesions are summarized in Tables 1 and 2.
Proper evaluation of the imaging findings combined with clinical evaluation yields diagnostic accuracy for distinguishing between benign and malignant perirectal cystic lesions.
Figures and Tables
Fig. 1
Tailgut cyst in an age 59 male. (a) Contrast-enhanced coronal CT scan shows a multilocular cystic lesion (*) without solid component in the perirectal region. (b) Photograph of the gross pathologic specimen of the tailgut cyst shows a welldemarcated, multilocular, cystic mass (*) without a solid component.

Fig. 2
Tailgut cyst in an age 54 male. (a, b) T1-weighted and T2-weighted axial MR image show a well-defined, thin-walled cystic mass with inhomogeneous signal intensity between the lower rectum and the coccyx (*).
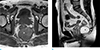
Fig. 3
Rectal abscess in an age 70 male who presented with fever and abdominal pain. (a) T2-weighted axial MR image shows a well-demarcated lesion with air-fluid level (*). (b) Contrast-enhanced axial CT scan shows capsular ring enhancement with contrast (arrow). Mild fat infiltration around the rectum also is observed.
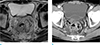
Fig. 4
Teratoma in an age 36 female. (a) T2-weighted axial MR image shows a well-defined cystic lesion (*) with unevenly surrounded wall (arrows). (b) Contrast-enhanced axial CT scan shows a lobulated cystic lesion with focal calcification in the wall (empty arrow).

Fig. 5
Epidermoid cyst in an age 65 female. (a, b) axial T2-weighted MR image shows a thin-walled lobulated lesion (*) with mainly high and overall heterogeneous signal intensity in the rectum.
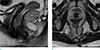
Fig. 6
A dermoid cyst in an age 48 female. (a, b) Sagittal and axial T2- and T1-weighted MR image show a thin-walled lobulated cystic mass (*) without internal solid portion. (c) Contrast enhanced T1-weighted MR image shows no visible enhancement in the cystic mass (*).
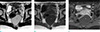
Fig. 7
Intramural hematoma of the rectum in an age 74 male who presented with hematochezia and treated by endoscopic clipping. (a) Non-enhanced axial CT scan shows a hyperdense lesion (*) with the endoscopic clip (arrow). (b) Contrastenhanced coronal CT image shows a non-enhanced lesion (*) and no contrast extravasation.
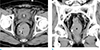
Fig. 8
Endoscopic diagnosis of a diffuse cavernous hemangioma in an age 41 male. (a) Contrast-enhanced axial CT image shows an eccentric wall thickening of the rectum (*) with some internal calcification (arrows) and low attenuating portions. (b) Colonoscopy shows numerous, prune-colored, polypoid lesions.
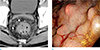
Fig. 9
Colitis cystica profunda in an age 67 male. (a) Contrast-enhanced axial CT scan shows edematous rectal wall thickening with a septated submucosal cystic lesion (arrow) in the posterior wall of the rectum. (b) The photograph of the gross pathologic specimen of colitis cystica profunda shows a well-demarcated submucosal cyst (*).
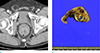
Fig. 10
Primary extracranial meningioma in an age 67 male. (a) Pre-enhanced axial CT scan shows a well-defined and lobulated mass with inhomogeneous low density that mainly consists of a cystic component (*). (b) Contrast-enhanced axial CT image shows enhancing peripheral areas and internal septa-like enhancement (black arrow). This mass is closely abutting to the rectum with maintained fat planes (white arrow).
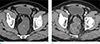
Fig. 11
Mucinous adenocarcinoma in an age 52 male who presented with anal pain. (a, b) Axial and sagittal T2-weighted MR image shows a lobulated, high signal intensity lesion around the rectum (*) and with invasion of the prostate (empty arrow), posterior wall of the urinary bladder (arrowheads), and right levator ani muscle (arrows).
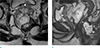
Fig. 12
Mucinous adenocarcinoma arising from a tailgut cyst in an age 66 male. (a) Contrast-enhanced axial CT scan shows a multiseptated cystic lesion with a thickened wall (empty arrow), rim calcification (arrowhead), and internal enhancing solid component (arrow). (b) Photograph of the gross pathologic specimen of the cystic lesion shows a well-demarcated multilocular cystic mass with an internal solid component (arrow).
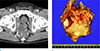
Fig. 13
An age 60 male with mucinous adenocrcinoma arising from a fistula-in-ano who presented with anal pain. (a) Axial T2-weighted MR image shows a trans-sphincteric fistula (arrow) and a multiloculated cystic lesion arising from the fistula (*). (b) Axial T1-weighted image shows a low signal intensity lesion that extends to the right gluteus muscle (empty arrow).
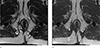
Fig. 14
Mucinous adenocarcinoma arising from mature teratoma in an age 61 female who presented with a palpable, coccygeal mass. (a, b) Axial and sagittal T2-weighted MR image shows a multiloculated, cystic lesion with heterogeneous signal intensity (*) that extends to the gluteus maximus (arrows) and coccyx (arrowheads).
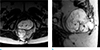
Fig. 15
Rectal GIST in an age 60 female. (a) Sagittal T2-weighted, MR image shows a mass abutting to the rectum with heterogeneous signal intensity and internal bright signal intensity (*). (b) Contrast-enhanced T1-weighted MR image shows an inhomogeneous enhancing mass with unenhanced cystic portion (*) that displaced the bladder anteriorly (arrow). (c) Contrast-enhanced axial CT scan shows a heterogeneously enhancing mass with internal cystic portion (*).
References
1. Lee J, Park CM, Kim KA, et al. Cystic lesions of the gastrointestinal tract: multimodality imaging with pathologic correlations. Korean J Radiol. 2010; 11:457–468.
2. Purysko AS, Coppa CP, Kalady MF, et al. Benign and malignant tumors of the rectum and perirectal region. Abdom Imaging. 2014; 39:824–852.
3. Yang DM, Park CH, Jin W, et al. Tailgut cyst: MRI evaluation. AJR Am J Roentgenol. 2005; 184:1519–1523.
4. Llauger J, Palmer J, Perez C, Monill J, Ribe J, Moreno A. The normal and pathologic ischiorectal fossa at CT and MR imaging. Radiographics. 1998; 18:61–82.
5. Guillaumin E, Jeffrey RB Jr, Shea WJ, Asling CW, Goldberg HI. Perirectal inflammatory disease: CT findings. Radiology. 1986; 161:153–157.
6. Afuwape OO, Ogundoyin OO, Ogunlana DI, Adeleye AO. Adult sacrococcygeal teratoma: a case report. Ghana Med J. 2009; 43:40–42.
7. Kumar N, Khosla D, Kumar R, Saikia UN, Singh S. Sacrococcygeal teratoma in adult: Two rare case reports and review of literature. Int J Appl Basic Med Res. 2014; 4:122–124.
8. Alvi MI, Mubarak F, Khandwala K, Barakzai MD, Memon A. A rare case of presacral epidermoid cyst in an adult male: Emphasis on diffusion weighted magnetic resonance sequences in preoperative imaging. Cureus. 2018; 10:e2050.
9. Kesici U, Sakman G, Mataraci E. Retrorectal/Presacral epidermoid cyst: report of a case. Eurasian J Med. 2013; 45:207–210.
10. Dahan H, Arrive L, Wendum D, Docou le Pointe H, Djouhri H, Tubiana JM. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics. 2001; 21:575–584.
11. Dwarkasing RS, Verschuuren SI, van Leenders G, Braun LMM, Krestin GP, Schouten WR. Primary cystic lesions of the retrorectal space: MRI evaluation and clinical assessment. AJR Am J Roentgenol. 2017; 209:790–796.
12. O'Malley CM, Remer EM, Delaney C. Imaging of the presacral space. Semin Colon Rectal Surg. 2004; 15:2–11.
13. Battal B, Kocaoglu M, Ors F, Akgun V, Tasar M. Obstructive rectal intramural hematoma caused by a foreign body. Emerg Radiol. 2009; 16:75–77.
14. Dhawan V, Mohamed A, Fedorak RN. Gastric intramural hematoma: a case report and literature review. Can J Gastroenterol. 2009; 23:19–22.
15. Sztarkier I, Benharroch D, Walfisch S, Delgado J. Colitis cystica profunda and solitary rectal ulcer syndrome-polypoid variant: Two confusing clinical conditions. Eur J Intern Med. 2006; 17:578–579.
16. Inan N, Arslan AS, Akansel G, Anik Y, Gurbuz Y, Tugay M. Colitis cystica profunda: MRI appearance. Abdom Imaging. 2007; 32:239–242.
17. Lang FF, Macdonald OK, Fuller GN, DeMonte F. Primary extradural meningiomas: a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000; 93:940–950.
18. Kyalakond K, Saini S, Rajagopal K, Karegowda LH. Radiological appearance of primary extracranial meningioma of the pelvis in a middle-aged woman. BMJ Case Rep. 2018; 2018.
19. Taori K, Kundaragi NG, Disawal A, et al. Imaging features of extra cranial parapharyngeal space meningioma: case report. Iran J Radiol. 2011; 8:176–181.
20. Rullier A, Laurent C, Vendrely V, Le Bail B, Bioulac-Sage P, Rullier E. Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma. Am J Surg Pathol. 2005; 29:602–606.
21. Hanski C. Is mucinous carcinoma of the colorectum a distinct genetic entity? Br J Cancer. 1995; 72:1350–1356.
22. Sjodahl RI, Myrelid P, Soderholm JD. Anal and rectal cancer in Crohn's disease. Colorectal Dis. 2003; 5:490–495.
23. Lee NK, Kim S, Kim HS, et al. Spectrum of mucin-producing neoplastic conditions of the abdomen and pelvis: cross-sectional imaging evaluation. World J Gastroenterol. 2011; 17:4757–4771.
24. Chhabra S, Wise S, Maloney-Patel N, Rezac C, Poplin E. Adenocarcinoma associated with tail gut cyst. J Gastrointest Oncol. 2013; 4:97–100.
25. Au E, Anderson O, Morgan B, Alarcon L, George ML. Tailgut cysts: report of two cases. Int J Colorectal Dis. 2009; 24:345–350.
26. Gonul , II , Baglan T, Pala I, Mentes B. Tailgut cysts: diagnostic challenge for both pathologists and clinicians. Int J Colorectal Dis. 2007; 22:1283–1285.
27. Johnson AR, Ros PR, Hjermstad BM. Tailgut cyst: diagnosis with CT and sonography. AJR Am J Roentgenol. 1986; 147:1309–1311.
28. Lim KE, Hsu WC, Wang CR. Ta i lgut cyst wi th malignancy: MR imaging findings. AJR Am J Roentgenol. 1998; 170:1488–1490.
29. Moulopoulos LA, Karvouni E, Kehagias D, Dimopoulos MA, Gouliamos A, Vlahos L. MR imaging of complex tail-gut cysts. Clin Radiol. 1999; 54:118–122.
30. Getz SB Jr, Ough YD, Patterson RB, Kovalcik PJ. Mucinous adenocarcinoma developing in chronic anal fistula: report of two cases and review of the literature. Dis Colon Rectum. 1981; 24:562–566.
31. Heidenreich A, Collarini HA, Paladino AM, Fernandez JM, Calvo TO. Cancer in anal fistulas: report of two cases. Dis Colon Rectum. 1966; 9:371–376.
32. Hama Y, Makita K, Yamana T, Dodanuki K. Mucinous adenocarcinoma arising from fistula in ano: MRI findings. AJR Am J Roentgenol. 2006; 187:517–521.
33. Kim JH, Lee TS, Oh HK, Choi YS. A case of mucinous adenocarcinoma arising from retroperitoneal teratoma treated with chemoradiation. J Gynecol Oncol. 2009; 20:126–128.
34. Chang YL, Wu CT, Lee YC. Mediastinal and retroperitoneal teratoma with focal gastrointestinal adenocarcinoma. J Thorac Oncol. 2006; 1:729–731.
35. Jiang ZX, Zhang SJ, Peng WJ, Yu BH. Rectal gastrointestinal stromal tumors: imaging features with clinical and pathological correlation. World J Gastroenterol. 2013; 19:3108–3116.
36. Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics. 2006; 26:481–495.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download