Abstract
Idiopathic pulmonary fibrosis (IPF) is a condition that has been described as alveolar collapse and thickening, which correlate with dysregulated surfactant production and injury to type 2 alveolar cells. As resolution of chest computed tomography has improved, especially with the development of high-resolution computed tomography (HRCT), the diagnostic measures adopted for pulmonary fibrosis has gradually shifted from biopsy to HRCT. This shift towards HRCT has aided in diagnostic evaluation and detection of the therapeutic and adverse effects of drugs for pulmonary fibrosis. Further, after the endpoint was changed to forced vital capacity, significant improvements are being observed in clinical trial outcomes. Currently active clinical trials are replacing lung biopsy with HRCT. In 2014, pirfenidone and nintedanib gained approval for tandem use in patients with IPF. These drugs were found to not only reduce the progression of pulmonary fibrosis, but also the acute exacerbation and mortality associated with the condition. These drugs showed consistent benefits regardless of the severity of patients’ symptoms. Additionally, both nintedanib and pirfenidone were found to be effective in patients with advanced pulmonary fibrosis that was not classified as IPF. Nintedanib has been shown to reduce forced vital capacity in interstitial lung diseases associated with systemic sclerosis. In the next three to five years, many changes in treatment are expected, not only for IPF, but also for the entire spectrum of pulmonary fibrotic diseases. Pirfenidone and nintedanib are now considered standard treatments for IPF and few other fibrotic lung diseases. Clinicians treating patients with pulmonary fibrosis should keep themselves updated with the results of clinical trials that are currently underway.
Go to : 
REFERENCES
1. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011; 183:788–824.

2. Albert RK, Schwartz DA. Revealing the secrets of idiopathic pulmonary fibrosis. N Engl J Med. 2019; 380:94–96.

3. Kim DS. Diagnostic approaches to diffuse interstitial lung diseases. J Korean Med Assoc. 2009; 52:5–13.

4. Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, Waldron J, Colby T, Muller N, Lynch D, Galvin J, Gross B, Hogg J, Toews G, Helmers R, Cooper JA Jr, Baughman R, Strange C, Millard M. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001; 164:193–196.

5. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, King TE Jr, Lancaster L, Noble PW, Sahn SA, Thomeer M, Valeyre D, Wells AU. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011; 184:1382–1389.
6. Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estépar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiríksdottír G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O'Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O'Connor GT, Washko GR, Rosas IO, Hunninghake GM. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016; 315:672–681.

7. Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, San José E; Estépar R, Schwartz DA, Rosas IO, Washko GR, OʼConnor GT, Hunninghake GM. Development and progression of interstitial lung abnormalities in the framingham heart study. Am J Respir Crit Care Med. 2016; 194:1514–1522.

8. Lee JW, Shehu E, Gjonbrataj J, Bahn YE, Rho BH, Lee MY, Choi WI. Clinical findings and outcomes in patients with possible usual interstitial pneumonia. Respir Med. 2015; 109:510–516.

9. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, Richeldi L, Kolb M, Tetzlaff K, Stowasser S, Coeck C, Clerisme-Beaty E, Rosenstock B, Quaresma M, Haeufel T, Goeldner RG, Schlenker-Herceg R, Brown KK. INBUILD Trial Investigators. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019; 381:1718–1727.

10. Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, Axmann J, Kirchgaessler KU, Samara K, Gilberg F, Cottin V. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2019 Sep 27. [Epub].https://doi.org/10.1016/S2213-2600(19)>30341-8.

11. Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, Raghu G, Sauter W, Girard M, Alves M, Clerisme-Beaty E, Stowasser S, Tetzlaff K, Kuwana M, Maher TM. SENSCIS Trial Investigators. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019; 380:2518–2528.

12. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schünemann HJ. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015; 192:e3–e19.

13. Carlos WG, Strek ME, Wang TS, Patel H, Raghu G, Wilson KC, Thomson CC. Treatment of idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2016; 13:115–117.

14. Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug treatment of idiopathic pulmonary fibrosis: systematic review and network metaanalysis. Chest. 2016; 149:756–766.
15. Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014; 58:13–19.

16. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2083–2092.

17. Costabel U, Albera C, Glassberg MK, Lancaster LH, Wuyts WA, Petzinger U, Gilberg F, Kirchgaessler KU, Noble PW. Effect of pirfenidone in patients with more advanced idiopathic pulmonary fibrosis. Respir Res. 2019; 20:55.

18. Yoon HY, Kim DS, Song JW. Efficacy and safety of pirfenidone in advanced idiopathic pulmonary fibrosis. Respiration. 2019; 97:242–251.

19. Tzouvelekis A, Ntolios P, Karampitsakos T, Tzilas V, Anevlavis S, Bouros E, Steiropoulos P, Koulouris N, Stratakos G, Froudarakis M, Bouros D. Safety and efficacy of pirfenidone in severe Idiopathic Pulmonary Fibrosis: A real-world observational study. Pulm Pharmacol Ther. 2017; 46:48–53.

20. Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, Sato A, Kudoh S. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005; 171:1040–1047.

21. Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, Takeuchi M, Raghu G, Kudoh S, Nukiwa T. Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010; 35:821–829.

22. Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015; 45:1434–1445.

23. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2071–2082.

24. Raghu G, Wells AU, Nicholson AG, Richeldi L, Flaherty KR, Le Maulf F, Stowasser S, Schlenker-Herceg R, Hansell DM. Effect of nintedanib in subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. Am J Respir Crit Care Med. 2017; 195:78–85.

25. Yoon HY, Park S, Kim DS, Song JW. Efficacy and safety of nintedanib in advanced idiopathic pulmonary fibrosis. Respir Res. 2018; 19:203.

26. Kolb M, Raghu G, Wells AU, Behr J, Richeldi L, Schinzel B, Quaresma M, Stowasser S, Martinez FJ. INSTAGE Investigators. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2018; 379:1722–1731.

27. Jeon K, Chung MP, Lee KS, Chung MJ, Han J, Koh WJ, Suh GY, Kim H, Kwon OJ. Prognostic factors and causes of death in Korean patients with idiopathic pulmonary fibrosis. Respir Med. 2006; 100:451–457.

28. Kondoh Y, Taniguchi H, Katsuta T, Kataoka K, Kimura T, Nishiyama O, Sakamoto K, Johkoh T, Nishimura M, Ono K, Kitaichi M. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010; 27:103–110.
29. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67–119.
30. Raghu G, Nathan SD, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Wells AU, Shao L, Zhou H, Henig N, Szwarcberg J, Gillies H, Montgomery AB, O'Riordan TG. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur Respir J. 2015; 46:1370–1377.

31. Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, Anstrom KJ, Martinez FJ. IPFnet Investigators. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013; 143:1699–1708.

32. Choi WI, Park SH, Park BJ, Lee CW. Interstitial lung disease and lung cancer development: a 5-year nationwide population-based study. Cancer Res Treat. 2018; 50:374–381.

33. Choi WI, Lee DY, Choi HG, Lee CW. Lung cancer development and mortality in interstitial lung disease with and without connective tissue diseases: a five-year Nationwide population-based study. Respir Res. 2019; 20:117.

34. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. 2016; 194:265–275.

35. Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, Snell GI, Verleden GM, Zamora MR, Glanville AR. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015; 34:1–15.

36. Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012; 366:1968–1977.
37. Raghu G, van den Blink B, Hamblin MJ, Brown AW, Golden JA, Ho LA, Wijsenbeek MS, Vasakova M, Pesci A, Antin-Ozerkis DE, Meyer KC, Kreuter M, Santin-Janin H, Mulder GJ, Bartholmai B, Gupta R, Richeldi L. Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA. 2018; 319:2299–2307.
38. Maher TM, van der Aar EM, Van de Steen O, Allamassey L, Desrivot J, Dupont S, Fagard L, Ford P, Fieuw A, Wuyts W. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med. 2018; 6:627–635.

39. Raghu G, Pellegrini CA, Yow E, Flaherty KR, Meyer K, Noth I, Scholand MB, Cello J, Ho LA, Pipavath S, Lee JS, Lin J, Maloney J, Martinez FJ, Morrow E, Patti MG, Rogers S, Wolters PJ, Yates R, Anstrom KJ, Collard HR. Laparoscopic antireflux surgery for the treatment of idiopathic pulmonary fibrosis (WRAP-IPF): a multicentre, randomised, controlled phase 2 trial. Lancet Respir Med. 2018; 6:707–714.

Go to : 
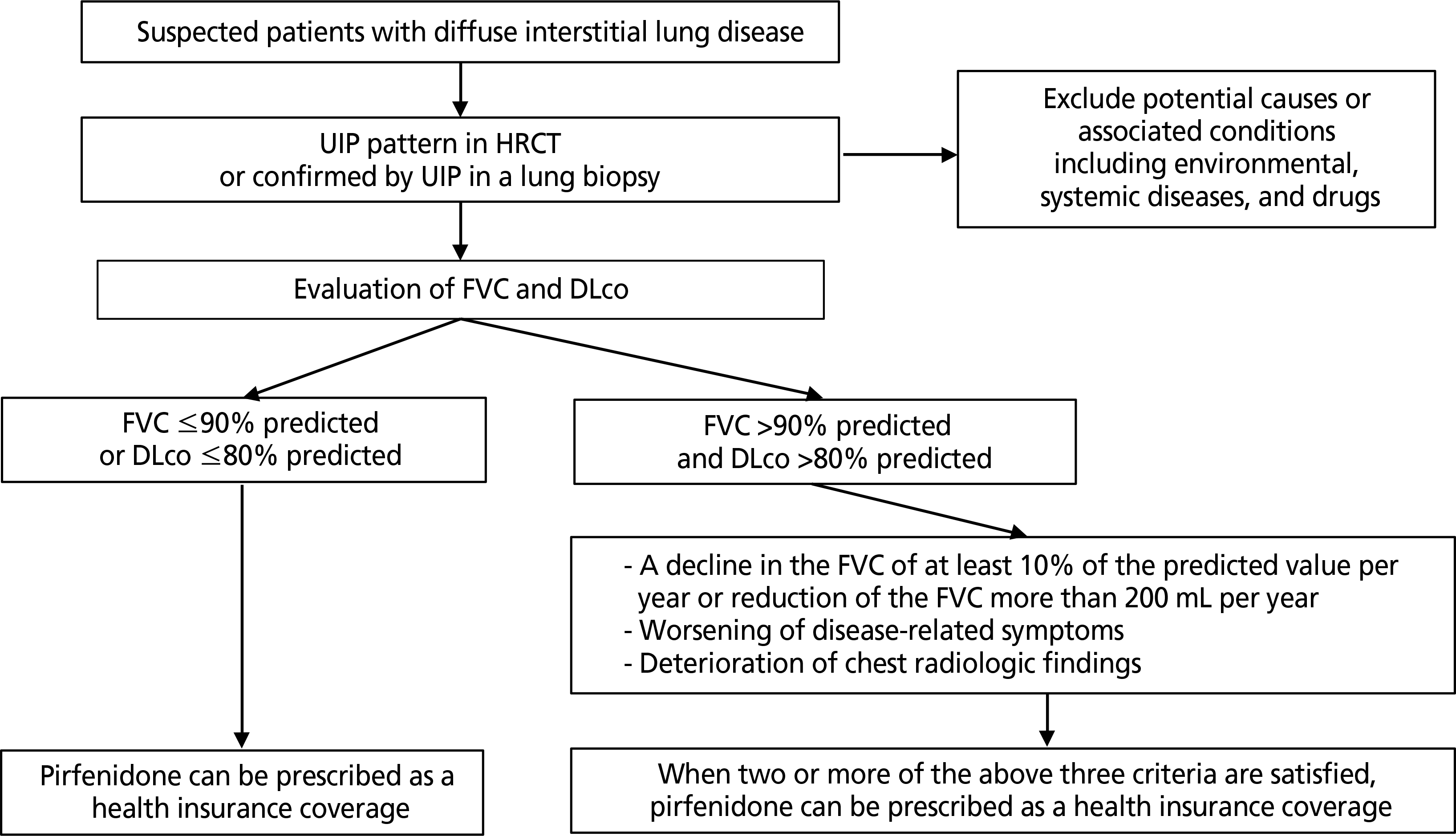 | Figure 1.Pirfenidone prescription algorithm based on the National Insurance Service payment in 2019. UIP, usual interstitial pneumonia; HRCT, high-resolution computed tomography; FVC, forced vital capacity; DLco, carbon monoxide diffusing capacity. |
Table 1.
Therapeutic effects of major drugs on IPF and other fibrotic interstitial lung diseases
| Fibrotic lung diseases |
Drugs |
||
|---|---|---|---|
| Pirfenidone | Nintedanib | Sildenafil | |
| IPF | |||
| Mild, FVC predicted ≥90% | + | + | |
| Moderate, FVC predicted ≥50% <90% | ++ | ++ | |
| Severe, FVC predicted <50% | + | + | |
| Possible usual interstitial pneumonia in HRCTa) | ? | + | |
| Unclassifiable ILD | + | + | |
| IPF with right ventricle dysfunction | ? | + | +b) |
| Chronic hypersensitivity pneumonitis | ? | + | |
| Nonspecific interstitial pneumonia | ? | + | |
| Systemic sclerosis-associated ILD | ? | ++ | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download