Aortoiliac occlusive disease (AIOD) is a peripheral arterial disease caused by atherosclerosis from the infra-renal artery to the iliac arteries. AIOD in renal transplant patients may limit the blood flow to the graft, resulting in symptoms and signs similar to Transplant renal artery stenosis (TRAS). Considering that atherosclerosis causes gradual vascular occlusion, it is necessary to distinguish it from TRAS.
Herein, we present a case of AIOD diagnosed in 17 years after transplantation with graft kidney dysfunction and refractory hypertension and treated with percutaneous angioplasty and stent graft insertion.
CASE
19 years ago, a 49-year-old woman underwent a non-related live kidney transplant for an unknown caused end-stage renal disease. For 14 years after the transplantation, there was no rejection and complications, and remained stable graft function in the baseline serum creatine of 0.8–1.1 mg/dL. There was a temporary rise in serum creatine at 1.97 mg/dL at 15 years and 1.64 mg/dL at 16 years postoperatively, but it stabilized soon after short-term intravenous saline administration. The baseline serum creatine was then maintained at 1.2–1.5 mg/dL, slightly higher than before, and the systolic blood pressure was maintained at 140 mmHg by taking four different type of antihypertensive drugs twice daily.
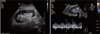
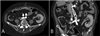
At 17 years postoperatively, the patient was hospitalized due to a sharp decrease in urine volume, general edema, shortness of breath, and an increase in serum creatine of 3.65 mg/dL. Duplex ultrasonography showed no change of the appearance and echogenicity of the graft kidney, but was measured by the pulsus parvus et tardus pattern and the reduced resistive index(RI) of 0.5 by the intrarenal artery doppler scan (Fig. 1). Suspecting graft kidney ischemia, CT angiography was performed to identify the site of stenosis. CT angiography presented bilateral common iliac artery stenosis (TASC classification C) (Fig. 2). The patient had no symptom of claudication, but performed an ankle-brachial index to quantify the degree of stenosis before and after treatment. The result of ABI was 0.73 on the right side and 0.50 on the left side of the transplanted kidney (normal value: 0.9–1.2).
Under local anesthesia, both common femoral artery approach was performed, and initial angiography was obtained via less stenotic lesion of Rt. access. The lesion of Lt. common iliac was neartotal occlusion, and the stenosis of right common iliac artery was up to 80% of the lumen. Upon the left approach, successful retrograde recanalization was done with .014” guide wire and it's supporting catheter. Pre-ballooning (3.5 * 20 mm, PTCA balloon, Nimbus PICO, BARD) was performed to smoothly deliver stent grafts to the lesion, and balloon expandable stent grafts (9 * 58 mm, Lifestream, BARD, 9 ATM) was inflated until the patient complained of pain. The lesions on the right also was deployed stent graft of the same size and length. The procedure was had completed after confirming patent artery of graft kidney limb without thromboembolism (Fig. 3). The next day, ABI rose to 0.95 on the right and 0.89 on the left.
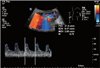
Six months after the procedure, the serum creatine level decreased to 0.97 mg/dL, and one antihypertensive drug was enough to make her blood pressure stable. Doppler waveform and resistance index of intra-renal artery were normalized (Fig. 4).
DISCUSSION
AIOD is a peripheral artery disease in which gradual occlusion of arterial blood due to atherosclerosis, which extends from the infra-renal aorta to the iliac arteries, limits blood flow to the lower extremities. Trans Atlantic Inter-Society Consensus Group (TASC), published by European and North American vascular specialists, has issued recommendations for the treatment and management of peripheral arterial disease. The proposed Trans-Atlantic Inter-Society Consensus (TASC II) guidelines are now widely used.1 TASC guideline states that TASC classification A, B are appropriate for interventional treatment, TASC classification C, D recommend surgical treatment. However, in the TASC classifications C and D, interventional procedures have been applied depending on the severity of the patient's comorbidity and on the patient's own choice. Since the publication of the TASC II Guideline in 2007, the development of interventional instruments and techniques has led to an increasing number of interventions as primary treatments in low risk groups. In low-groups, interventions are often used as the primary treatment.2 Recent COBEST study conducted in 2016 suggested the use of stent grafts in TASC classification C and D lesions.3
AIOD in renal transplant patients is a progressive obstruction of the recipient's artery due to atherosclerosis and should be distinguished by timing and lesion location from the TRAS that occurs at the anastomotic site at 6 months postoperatively.4 Considering the 15-20 year lifespan of the graft, a decrease in graft function due to gradual closure of inflow artery may be underestimated as a natural graft kidney failure.5
If the inflow of the transplant kidney is restricted due to AIOD, the renin-angiotensin-aldosterone system may be activated, resulting in symptoms and signs of refractory hypertension, water retention, and elevated creatine in the blood. However, the gradual development of the collateral circulation into the lower extremities may not appeal to the symptoms of intermittent claudication.6 Some groups emphasize that refractory hypertension due to graft kidney ischemia is the main indication of disease.7
Duplex ultrasonography should be the first choice in screening and then angiography should be performed to confirm a location of the lesion.4 Because of the heavy calcification of the lesion, CT angiography without the use of contrast media can also diagnose a location of the lesion. ABI is a non-invasive screening test for peripheral arterial disease, which is relatively accurate and economical indirectly assessing the vascular status of patients. ABI for diagnosing peripheral arterial disease is 80% or higher in sensitivity and specificity. A number of guidelines suggested that further diagnostic tests are needed to differentiate peripheral arterial disease when ABI is less than 0.9. It can also be useful for comparison before and after treatment and follow-up.8




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download