Abstract
Tracheostomy is increasingly performed in children for upper airway anomalies. Here, an 18-month-old child (height 84.1 cm, weight 12.5 kg) presented to the emergency department with dyspnea, stridor, and chest retraction. However, exploration of the airways using a bronchoscope failed due to subglottic stenosis. Therefore, a surgical tracheostomy was successfully performed with manual mask ventilation. However, pneumomediastinum was found in the postoperative chest radiograph. Although an oxygen saturation of 99% was initially maintained, oxygen saturation levels dropped, due to sudden dyspnea, after 3 hours. A chest radiograph taken at this time revealed a left tension pneumothorax and small right pneumothorax. Despite a needle thoracostomy, the pneumothorax was aggravated, and cardiac arrest occurred. Cardiopulmonary-cerebral resuscitation was performed, but the patient was declared dead 30 minutes later. This study highlights the fatal complications that can occur in children during tracheostomy. Therefore, close monitoring, immediate suspicion, recognition, and aggressive management may avoid fatal outcomes.
Tracheostomy is increasingly being performed in children, leading to improvements in neonatal and pediatric ICU care; it is frequently performed in children with upper-airway anomalies. However, there are several complications associated with tracheostomy. Some early complications include air leaks, injury to surrounding tissues, pulmonary edema, respiratory arrest, airway obstruction, and injury caused by tube placement. Delayed complications include airway obstruction, tracheoesophageal fistula, and swallowing problems, some of which can be life-threatening in children.1 Here, we report a case of a fatal bilateral pneumothorax complication with surgical tracheostomy in an 18-month-old child.
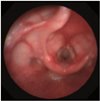
An 18-month-old child (height 84.1 cm, weight 12.5 kg) presented to the emergency department with dyspnea, stridor, and chest retraction. Symptoms aggravated despite treatment with a nebulizer and steroid. Computed tomography was performed after admission where no foreign bodies were found. A flexible bronchoscope was used for exploration of the airways by the pediatric resident. However, the presence of subglottic stenosis made advancing the scope into the trachea, via the vocal cord, difficult (Fig. 1). The severity of the patient's dyspnea increased. Therefore, we decided to use a rigid ventilating bronchoscope for exploration and to perform tracheostomy with controlled ventilation under general anesthesia.
When the patient was admitted to an operation room, initial vital signs showed a blood pressure of 115/72 mm Hg, a heart rate of 114 beats/min, a respiratory rate of 32/min, and an oxygen saturation of 92%. After induction of anesthesia with 25 mg of ketamine and 5 mg of rocuronium, the otolaryngologist attempted to insert a rigid bronchoscope (Karl Storz, Tuttlingen, Germany) with an outer diameter of 4.0 mm. However, insertion failed beyond the subglottic stenosis. The patient displayed a Cormack-Lehane classification grade of 1, and intubation was attempted using a wire-reinforced endotracheal tube (Mallinckrodt ReinforcedTM, Covidien, Dublin, Ireland) with an inner diameter of 3.5 and 4.0 mm. However, the endotracheal tube failed to pass the vocal cord. We did not make an additional attempt due to the surrounding tissue edema and risk of complete airway obstruction. We therefore decided to perform a tracheostomy with manual mask ventilation for the management of dyspnea. Anesthesia was maintained with inhalational sevoflurane. Surgical tracheostomy was performed with manual mask ventilation at set adjustable pressure-limiting pressure levels of 10 cmH2O, and a 4.5-mm tracheostomy tube was inserted by an otolaryngologist. The duration of the surgery was 55 minutes, and no hypoxic events occurred during anesthesia.
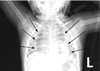
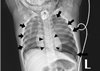
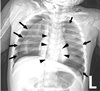
At the post-anesthesia care unit following surgery, oxygen was supplied via a T-piece at about 2 L/min and an oxygen saturation of 100% was maintained. The patient was spontaneously breathing, with improved symptoms of dyspnea. However, a pneumomediastinum was found in the postoperative chest radiograph (Fig. 2). At the ward, an oxygen saturation of 99% was maintained with oxygen supply via T-piece about 2 L/min for 3 hours. However, sudden dyspnea occurred and oxygen saturation levels dropped to 88%. After manual ventilation, oxygen saturation rose to 99% temporarily, dyspnea occurred once again and oxygen saturation dropped to 92%. A blood clot was found in the tracheostomy tube. Thus, the clot was removed. However, suction catheter insertion was not easy even after removing the clot, and the tracheostomy tube was changed. The 4.5-mm tracheostomy tube was removed due to concerns regarding tracheal wall narrowing, and a 4.0-mm tracheostomy tube was inserted by an otolaryngology resident. A chest radiograph taken at this time revealed a left tension pneumothorax and small right pneumothorax (Fig. 3). Needle thoracostomy was performed by a pediatric resident using a 20-gauge angiocatheter needle, and 200 cc of air was aspirated. The following chest radiograph showed the existing left pneumothorax, but an aggravated right pneumothorax (Fig. 4). During tube thoracostomy, which was performed by a thoracic surgeon, the pulse rate decreased to 70 beats/min, with an oxygen desaturation of up to 45~55%. CPCR was performed. Epinephrine 120 mcg was injected every 5 min, and 10 mEq of sodium bicarbonate was injected thrice. But the patient was declared dead 30 minutes later.
Many complications can arise from tracheostomy being performed in children. For example, pneumomediastinum can occur in children who have undergone tracheostomy, through dissection of air between the deep and superficial cervical fascia. In order to prevent pneumomediastinum, minimizing the pretracheal and paratracheal dissection is essential.1 In the absence of associated comorbidities, spontaneous pneumomediastinum can be managed with observation without the need for further imaging.2 In our case, we failed to consider the possibility of tracheal injury despite the presence of pneumomediastinum in the postoperative chest radiography. Therefore, we overlooked the fact that the pneumomediastinum could be worsened by continuous manual ventilation. Pneumothorax most commonly occurs due to an increase in the pressure gradient between the mediastinum and pleural space. Presence of pneumomediastinum allows gas to rupture the mediastinal pleura, causing it to enter the pleural space.3 Air from the mediastinum can migrate to the pericardium and cause pneumothorax and subcutaneous emphysema.4 The most efficient mechanism that has been described to prevent the risk of developing pneumothorax involves maintaining the peak inspiratory pressures low.5 In this situation, a fiberoptic bronchoscopic assessment is recommended to identify the site and severity of the injury. While a small tracheal injury can be managed conservatively, a larger tear of over 1 cm requires surgical repair.6 Additionally, using the fiberoptic bronchoscope as a guide, prevents the tracheostomy catheter being inserted into a false cavity. This procedure can also prevent tracheal wall injury caused by the tracheostomy tube.7 Therefore, tracheostomy guided by a fiberoptic bronchoscope is recommended in pediatric patients.
In our case, the pneumothorax was thought to develop from pneumomediastinum, and was exacerbated by manual ventilation after desaturation. Moreover, the blood clot in the tracheostomy tube could have cause airway obstruction and aggravated the pneumothorax. Tension pneumothorax occurs when a one-way valve is created between the lung and pleura. Air accumulates in the pleural cavity with every breath, and intrapleural pressure is elevated. This leads to a shrinkage in the ipsilateral lung, and the mediastinum is pushed to the opposite side. This reduces venous return, resulting in a cardiac arrest. During tension pneumothorax, chest radiological findings show a hemi-diapragmatic depression, increased rib separation, increased thoracic volume, ipsilateral flattening at the heart border, and a contralateral mediastinal deviation.189 In our case, tension pneumothorax, where the ipsilateral hemidiaphragm is pushed down was not shown in the initial postoperative chest radiograph, but instead was found later (Fig. 2, 3). In such case, a prompt decompression is crucial wherein a needle thoracostomy followed by a tube thoracostomy must be performed. The site for needle thoracostomy is generally the midclavicular line of the second rib in the anterior chest. Recently, the American College of Surgeons has recommended use of the fourth or fifth intercostal space at the anterior axillary line, at the same location used for a tube thoracostomy.10 However, several problems have been identified regarding needle thoracostomy, such as inappropriate length of the needle, its inability to puncture through the chest wall, catheter being tangled, and drainage failure. Needle thoracostomy needs to be repeated before adequate decompression is confirmed and a chest tube is inserted.811 In our case, after the needle thoracostomy, decompression was not enough in the left pneumothorax. Moreover, the right pneumothorax worsened due to manual ventilation. Finally, a cardiopulmonary collapse occurred, and the patient showed hypotension, bradycardia, and desaturation. Despite CPCR, the patient was declared dead after 30 minutes.
In conclusion, fatal complications can occur in children during tracheostomy. Complications such as posterior tracheal wall injury, pneumomediastinum, pneumothorax, and cardiac arrest can develop. Pneumomediastinum, particularly developed after tracheostomy, needs to be monitored closely and treated aggressively. While performing a tracheostomy, regardless of whether it is a surgical tracheostomy or percutaneous dilatational tracheostomy, use of a fiberoptic bronchoscope can prevent life-threatening complications like tracheal wall injury. Inadvertent manual ventilation can also aggravate the situation, especially in a child. Therefore, immediate suspicion of potential complications and aggressive management, such as thoracostomy, can reduce fatal outcomes.
Figures and Tables
References
2. Fitzwater JW, Silva NN, Knight CG, Malvezzi L, Ramos-Irizarry C, Burnweit CA. Management of spontaneous pneumomediastinum in children. J Pediatr Surg. 2015; 50:983–986.

3. De la Matta-Martín M, Galán MJ, Gallego J. Pneumomediastinum and pneumothorax during emergency tracheotomy under spontaneous ventilation: Macklin meets Müeller? Rev Esp Anestesiol Reanim. 2016; 63:231–234.

4. Gasser CR, Pellaton R, Rochat CP. Pediatric Spontaneous Pneumomediastinum:Narrative Literature Review. Pediatr Emerg Care. 2017; 33:370–374.
5. El-Nawawy AA, AI-Halawany AS, Antonios MA, Newegy RG. Prevalence and risk factors of pneumothorax among patients admitted to a pediatric intensive care unit. Indian J Crit Care Med. 2016; 20:453–458.

6. Gupta P, Modrykamien A. Fatal case of tension pneumothorax and subcutaneous emphysema after open surgical tracheostomy. J Intensive Care Med. 2014; 29:298–301.

7. Shen G, Yin H, Cao Y, Zhang M, Wu J, Jiang X, et al. Percutaneous dilatational tracheostomy versus fibre optic bronchoscopyguided percutaneous dilatational tracheostomy in critically ill patients: a randomized controlled trial. Ir J Med Sci. 2019; 188:675–681.

8. Leigh-Smith S, Harris T. Tension pneumothorax – time for a re-think? Emerg Med J. 2005; 22:8–16.
10. Laan DV, Vu TD, Thiels CA, Pandian TK, Schiller HJ, Murad MH, et al. Chest wall thickness and decompression failure: A systematic review and meta-analysis comparing anatomic locations in needle thoracostomy. Injury. 2016; 47:797–804.

11. Jones R, Hollingsworth J. Tension pneumothoraces not responding to needle thoracentesis. Emerg Med J. 2002; 19:176–177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download