Abstract
Objectives
Staphylococcal scalded skin syndrome (4S), a blistering dermatosis caused by exfoliative toxins from Staphylococcus aureus, occurs frequently in patients with atopic dermatitis (AD). However, association between 4S and AD has not rarely been reported. We investigated the characteristics of 4S according to AD status.
Methods
The study included 146 children with 4S who visited Busan St. Mary's Hospital from 2007–2018. Clinical features were analyzed from medical records and pictures, and 4S was classified as localized or generalized. We also retrospectively investigated the preceding conditions and test results related to AD.
Results
Among 146 patients with 4S, median age was 2.0 years, and 35 (24.0%) had AD. Since 2007, the incidence of both 4S and AD have increased, without obvious seasonal patterns. Generalized and localized disease occurred in 90 and 56 patients, respectively. Twenty-four of 35 patients with AD (68.6%) and 32 of 111 (28.8%) without AD had localized disease. Significant differences were observed between the groups (P = 0.000). Among those with AD, the most common preceding condition was skin infection or unknown (45.2%); however, respiratory disease was the most common (47.9%) among patients without AD. Eosinophil levels were higher in the AD group (P = 0.002), and there were no statistically significant differences in total immunoglobulin E (IgE), Dermatophagoides farinae (Df IgE), egg-white IgE, and culture results between the groups.
Staphylococcal scalded skin syndrome (4S), caused by epidermolytic toxin and exfoliative toxins A and B produced by Staphylococcus aureus, is a major pathogen of pyogenic skin infection that binds to Desmoglein-1 through blood circulation, severing the connection between skin and causing skin exfoliation. It is characterized by scarlet fever-like tender erythema, which may occur in patients younger than 5 years of age, with symptoms such as sagging, fever, irritability, and which typically develops in the flexures, eyes, nose, and mouth. 4S is accompanied by blisters, erosions, and skin exfoliation and is classified as localized or generalized subtypes depending on the presence or absence of Nikolsky sign and the range of affected skin surface area.1 The preceding condition is primarily respiratory diseases but the condition may include conjunctival, umbilical, skin, or digestive diseases. Recently, the incidence of 4S in Korea has been increasing and there are reports that it is often accompanied by AD.23 Atopic dermatitis (AD) is a common chronic skin disease and the presence of the S. aureus is frequently detected on skin affected with AD. This study was conducted in children with 4S visiting a single medical institution over the past 12 years and aimed to investigate the characteristics associated with the development of 4S in AD.
The study included 146 children diagnosed with 4S who visited Busan St. Mary's Hospital in Jan. 2007 – Dec. 2018.
We retrospectively reviewed the medical records, photographs, and test results of the patients and examined the presence of AD; preceding conditions before 4S onset such as respiratory disease, skin infection like impetigo, newborn, etc; and the total immunoglobulin E (IgE), specific IgE, and eosinophil counts related to AD status and severity.45 Depending on the clinical features of 4S, the patients were classified as having either the generalized or localized subtype. The generalized subtype was defined as the presence of systemic tender erythema and blister except for the mucous membranes, positivity for Nikolsky sign, and involvement of more than 30% of the whole body area. The localized subtype had tender erythema and blister in characteristic locations such as the flexures, eyes and, mouth; Nikolsky sign negativity, and involvement of less than 30% of the whole body area.1 Several Pediatric allergy respiratory subspecialists diagnosed and classified skin lesions in characteristic locations with tender erythema, blisters, and skin exfoliation.
In culture tests, specimens were collected from throat, and skin lesions around the mouth and eyes of 4S patients using a cotton swab by medical staff wearing gloves. The specimens were placed in transport medium, transferred to a laboratory, inoculated into a blood agar medium, and cultured at 35℃ in 3–5% CO2 for 24 hours. When characteristic colonies were observed, S. aureus was directly identified using catalase and coagulase tests or by automated identification method using a VITEC2 instrument (Biomériusinc, Durham, North Carolina, USA). The cultures were inoculated with the identified bacteria coated on an 87 × 15 mm petri dish.
Cross tabulation analysis was performed using IBM SPSS version 16.0, and Pearson chi-square tests or Fisher's exact tests were used for comparison of categorical variables. Continuous variables were analyzed by Mann-Whitney test. P-values less than 0.05 were considered statistically significant.
Of the 146 patients diagnosed with 4S during the study period, 35 (24.0%) were diagnosed with AD and 69 (47.3%) were male. The median ages of patients with and without AD were 2.8 and 2.4 years, respectively, the difference was not statistically significant (P = 0.252). There were five newborns within one month from birth, and they are all generalized subtypes and was classified as non-AD because of diagnostic uncertainty of AD. There was no difference in 4S occurrence according to sex, and the difference with and without AD was also not statistically significant (P = 0.859).
There were fewer than 10 pediatric patients with 4S per year from 2007–2011; the number of patients has exceeded 10 since 2012. 2017 had the highest number of patients (29 patients). The numbers of pediatric patients with AD has also increased accordingly (Fig. 1).
Regarding the monthly distributions of cases, there were many patients in the winter months of December (18 patients), January (11 patients), and February (19 patients); fewer cases in the spring months of March (11 patients), April (8 patients), May (5 patients), and June (6 patients); and increasing number between summer and before winter in July (15 patients), August (13 patients), September (16 patients), October (15 patients), and November (9 patients). From summer to winter, the onset of 4S was increased compared to spring, but the onset of 4S in AD patients was sporadic across the year but a constant ratio was maintained apart from June and December (Fig. 2).
A total of 56 (38.4%) and 90 (61.6%) patients had localized and generalized subtypes, respectively. Of 35 patients with AD, 24 (68.6%) corresponded to localized subtype and 11 (31.4%) to generalized subtype. Of 111 patients without AD, 32 were localized subtype (28.8%) and 79 were generalized subtype (71.2%). The AD patients had more cases with localized subtypes than the patients without AD, and the differences was statistically significant (P = 0.000).
In throat culture, S. aureus was detected in 47 patients (32.2%). Among AD patients, S. aureus was detected in 8 (22.9%) of 35 patients Among patients without AD, S. aureus was detected in 39 (35.1%) of 111. There was no statistically significant difference in these rates of detection (P = 0.216).
In skin culture, S. aureus was detected in 97 patients (66.4%), more than were detected by throat culture. In AD patients, S. aureus was detected in 27 (77.1%) of 35 patients. In patients without AD, S. aureus was detected in 70 (63.1%) of 111 patients. There was no statistically significant difference in these rates of detection (P = 0.142).
The most common preceding condition was respiratory disease, which was present in 70 (47.9%) patients, including 11 (31.4%) of 35 AD patients and 59 of 111 (53.2%) patients without AD. Significantly fewer AD patients had respiratory disease as an preceding condition (P = 0.033).
The second most common preceding condition was the occurrence of skin lesions in characteristic sites and skin condition or unknown preceding condition were reported in 66 patients (45.2%), including 23 (65.7%) of 35 AD patients and 43 (38.7%) of 111 patients without AD, a statistically significant difference (P = 0.006) (Table 1).
Except for 10 patients whose preceding condition were digestive diseases (5 patients) or newborn (5 patients), Patients without respiratory diseases were included in the same category. Because the first symptom of 4S was skin lesions around the mouth and eyes and was ambiguous, it was difficult to distinguish from skin diseases such as impetigo.
There were no statistically significant differences in total IgE, Dermatophagoides farinae (Df)-specific IgE, or egg-white-specific IgE levels (P = 0.562, P = 0.727, and P = 0.385, respectively). However, there was a significant difference between groups in eosinophil counts, with mean concentrations of 617 cells/µL in AD patients and 387 cells/µL in patients without AD (P = 0.002) (Table 2).
4S is caused by exfoliative toxins secreted by S. aureus.6 In children, due to kidney immaturity, the exfoliative toxin A, B produced by S. aureus is not excreted smoothly and antibody production is low; thus, 4S appears to be more common in children than in adults.78
Although it is a relatively rare infectious disease, contrary to the trend of decreasing incidence of bacterial diseases due to improvement in public hygiene and environment along with development in medicine, the incidence of 4S is steadily increasing not only in Korea but also in other countries,291011 for reasons that are not clearly understood. 4S usually occurs in the autumn and winter months when the incidence of respiratory infections is increased1213 and in this study also, it increased from July and decreased only after March. The seasonal changes in AD patients were not significant and consistently occurred at a constant rate throughout the year.
In 2004, Kang et al.,14 reported that 64.0% and 36% of patients had generalized and localized subtypes, respectively, comparable to the 61.1% and 38.9%, respectively, observed in the present study. Although respiratory infection is reported to be the most common preceding condition,1213 for localized subtype, skin conditions such as impetigo have been reported in multiple cases by Hubiche et al.,15 Bae et al.,16 Elias et al.,17 and in other domestic studies.
S. aureus is a major pathogen of hospital- and community-acquired infections. It forms colonies in the mucous membranes of the nose, eyes, mouth, and flexures in 35–40% of normal people.18 In addition, more than 90% of colony formation is confirmed in the presence of skin conditions such as AD. This is related to the impairment of innate immunity observed in AD, leading to tissue invasion and cell damage, eventually causing chronicization and symptom aggravation. It was thought that AD patients were partially immune to exfoliative toxins and had antibodies through previous S. aureus exposure and therefore developed the minor or localized form of 4S.19 However, the association of localized 4S and AD had rarely been reported. A better understanding of mechanism may contribute to the development of improved therapeutic strategies for this complex disease.
Also, an increased number of eosinophils was observed in 4S patients with AD, a finding consistent with reports that eosinophil number is an indicator of the severity of acute phase and the positive correlation with the severity of skin symptoms in AD.520 However, as total and specific IgE levels did not differ, eosinophils may play an additional role in the development of 4S in AD patients.
The strength of this study is that the number of pediatric patients is larger than those of previous studies and, thus, it demonstrates the specificity of 4S in AD patients. However, this retrospective study relied on medical records and photographs and many elements of 4S and preceding condition were subjecting and diagnosed according to the clinical findings. It is important to distinguish between impetigo caused by S. aureus and tender erythema. Impetigo has no tender erythema, skin exfoliation, and spread of the toxin by blood circulation. In the present study, but it was challenging to distinguish between impetigo and 4S because the mean age of the patients was 2 years and tenderness was difficult to judge. These aspects should be improved and supplemented in a future prospective study.
In conclusion, 4S, which commonly develops in pediatric patients with AD, mainly occurred as the localized subtype and there were many cases in which the preceding conditions were unclear or with skin condition as the first symptom. This pattern differs from other cases of 4S in which respiratory diseases were the preceding conditions and showed the generalized subtype. Additional studies involving toxins such as exfoliative toxins and antibodies against them, which were not covered in this study, could help to clarify the association between AD and 4S.
Figures and Tables
 | Fig. 1Yearly distribution of the patients with staphylococcal scalded skin syndrome between 2007 and 2018 |
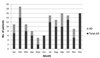 | Fig. 2Monthly distribution of the patients with staphylococcal scalded skin syndrome between 2007 and 2018 |
References
1. Berk DR, Bayliss SJ. MRSA, Staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010; 39:627.

2. Park CH, Na SR, Cho HM, Yoo EJ, Jung K, Kim EY, et al. Clinical features and the associated factors of staphylococcal scalded skin syndrome during the recent 10 years. Korean J Pediatr Infect Dis. 2008; 15:152–161.

3. Heo SY, Song YJ, Kim SJ, Park SY, Kang DC, Ma SH. A clinical review of community acquired methicillin resistant staphylococcal scalded skin syndrome. Korean J Pediatr Infect Dis. 2007; 14:83–90.

4. Lee JS, Kim TH, Cho GL, Jung JA, Kim JH. The classification between IgE and non-IgE mediated atopic dermatitis in Korean children. Pediatr Allergy Respir Dis. 2005; 15:352–358.

5. Uehara M, Izukura R, Sawai T. Blood eosinophilia in atopic dermatitis. Clin Exp Dermatol. 1990; 15:264–266.

6. Melish ME, Glasgow LA. The staphylococcal scalded-skin syndrome: development of an experimental model. N Engl J Med. 1970; 282:1114–1119.
7. Manders SM. Toxin-mediated streptococcal and staphylococcal disease. J Am Acad Dermatol. 1998; 39:383–398.

8. Iwatsuki K, Yamasaki O, Morizane S, Oono T. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006; 42:203–214.

9. Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014; 28:1418–1423.

10. Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in U.S. Children. Br J Dermatol. 2018; 178:704–708.

11. Arnold JD, Hoek SN, Kirkorian A. Epidemiology of staphylococcal scalded skin syndrome in the United States: A cross-sectional study, 2010-2014. J Am Acad Dermatol. 2018; 78:404–406.

12. Park JW, Hwang DK, Yu HJ. Staphylococcal scalded skin syndrome, review of 20 cases. Korean J Dermatol. 2002; 40:1051–1057.
13. Lee SH, Choi WK, Jung CH, Chung CJ, Lee DJ. Staphylococcal scalded skin syndrome in children: comparison of the clinical features of that isolated methicilin-resistant and methicillin-sensitive Staphylococcus aureus. Korean J Pediatr Infect Dis. 2004; 11:183–191.

14. Kang JD, Park SD. Reclassification of staphylococcal scalded skin syndrome by clinical analysis of 25 cases. Korean J Dermatol. 2004; 42:398–405.
15. Hubiche T, Bes M, Roudiere L, Langlaude F, Etienne J, Del Giudice P. Mild Staphylococcal scalded skin syndrome: an underdiagnosed clinical disorder. Br J Dermatol. 2012; 166:213–215.

16. Bae SH, Lee JB, Kim SJ, Lee SC, Won YH, Yun SJ. Case of bullous impetigo with enormous bulla developing into staphylococcal scalded skin syndrome. J Dermatol. 2016; 43:459–460.

17. Elias PM, Levy SW. Bullous impetigo: Occurrence of localized scalded skin syndrome in an adult. Arch Dermatol. 1976; 112:856–858.

18. Dancer SJ, Noble WC. Nasal, axillary, and perineal carriage of Staphylococcus aureus among women : identification of strains producing epidermolytic toxin. J Clin Pathol. 1991; 44:681–684.

19. Goolamali SI, Haddadeen C, Creamer JD, Hay R, Higgins EM, Morris-jones R. Localized staphylococcal scalded skin syndrome in five patients with atopic dermatitis. Br J Dermatol. 2010; 163:41–42.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download