Abstract
The latest definition of a prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit”; it now includes non-food elements and is applicable to extra-intestinal tissues. Prebiotics are recognized as a promising tool in the promotion of general health and in the prevention and treatment of numerous juvenile diseases. Prebiotics are considered an immunoactive agent, with the potential for long-lasting effects extending past active administration of the prebiotic. Because of its extremely low risk of serious adverse effects, ease of administration, and strong potential for influencing the composition and function of the microbiota in the gut and beyond, the beneficial clinical applications of prebiotics are expanding. Prebiotics are the third largest component of human breast milk. Preparations including galactooligosaccharides (GOS), fructooligosaccharides (FOS), 2'-fucosyllactose, lacto-N-neo-tetraose are examples of commonly used and studied products for supplementation in baby formula. In particular, the GOS/FOS combination is the most studied. Maintaining a healthy microbiome is essential to promote homeostasis of the gut and other organs. With more than 1,000 different microbial species in the gut, it is likely more feasible to modify the gut microbiota through the use of certain prebiotic mixtures rather than supplementing with a particular probiotic strain. In this review, we discuss the latest clinical evidence regarding prebiotics and its role in gut immunity, allergy, infections, inflammation, and functional gastrointestinal disorders.
Go to : 
Centuries ago, Hippocrates stated that “All diseases begin in the gut”. This statement has stood the test of time and has proven to be largely insightful. An increasing number of chronic non-communicable disorders affecting various bodily systems are linked to disturbances in the gut microbiome and originate early on in life. Along with genetic and environmental factors, diet is recognized as a key factor in shaping the composition and the function of the gut microbiome. Prebiotics are dietary fibers that can significantly influence the development of the microbial community in the gut. This review will discuss the relationship between prebiotics and the microbiome and the role of the gut-associated immune system in shaping gastrointestinal function. It will also sum up the evolution of the prebiotic concept and highlight their therapeutic potential during development.
Go to : 
By virtue of harboring about two thirds of the overall immune tissues and more than three quarters of the immunoglobulin producing cells, the gastrointestinal tract (GI) is considered the largest immune organ in the human body [12]. The gut-associated lymphoid tissue plays a key role in the complex mechanisms of immune regulation through a dynamic interaction with the GI tract. Upon exposure to microbe-related antigens, such interactions can result in either tolerance or elimination of the foreign antigens. Further, the presence of these antigens may promote inflammation of the GI tract resulting in compromised gut function and increased intestinal permeability [3]. A healthy intestinal microbiome is essential for homeostasis in the gut and in overall health; however, the uncontrolled excessive growth of certain bacterial populations leads to a variety of harmful conditions. The gut microbiota in a eubiotic status is characterized by a preponderance of potentially beneficial species. Disorders such as obesity, inflammatory bowel diseases, metabolic syndrome, allergy, autoimmune disorders, and autism are increasingly linked to dysbiosis in the gut [456]. This in turn leads to disturbances in the immune function of gut-associated lymphoid tissue (GALT) and associated damage of the GI.
The gut microbiota is increasingly recognized as an important factor influencing GALT immune function and with regard to its interactions with the gut epithelium. In the last few decades, knowledge of the gut microbiota has significantly expanded. More is known about its composition and function, with the evolution of new data targeting its modification in favor of promoting overall human health. Owing to its presence in large numbers (almost more than 10 times the number of cells in human body) and its genetic coding material (the microbiome, which is 150 times larger than the human genome), the gut microbiota is thought to have a profound effect on human metabolism and immune system development [7]. With such a well-defined functional capacity, there are emerging calls for it to be considered as an organ capable of interacting with other organs [8].
Go to : 
The majority of molecular testing based data suggests that babies are born germ free [9]. It is believed that a baby may acquire microbes prior to delivery via transplacental transfer; however emerging evidence negates this and in fact, suggests that the microbes were acquired through contamination [1011]. With the development of better detection techniques, the identification of fetal microbes will be feasible and this will improve current knowledge pertaining to GI development.
There are numerous factors including genetics, mode of delivery, host physiology, breast or bottle feeding and other environmental elements such as living conditions and use of medications that can influence the development of the gastrointestinal microbiota during the early stages of life [12131415].
Originating from the birth canal, Escherichia coli populates infant GI tracts early on and is followed by the appearance of bifidobacteria, Bacteroides, and Clostridium within the first week of life [16]. Bifidobacterium and Lactobacillus species tend to dominate the gut of breast milk-fed infants at the expense of E. coli and other facultative anaerobic microorganisms. In formula fed infants, Bacteroides, Clostridium, and Enterobacteriaceae cohabitate the gut and eventually eliminate bifidobacteria [171819]. After dynamic fluctuation in bacterial composition in the interim period, the gut microbiota become well established, functionally stable and resemble the composition observed in adults by around the age of 2–3 years [2021].
Go to : 
Diet is increasingly recognized as a key environmental factor that can modulate the composition and metabolic function of the GI microbiota [22]. The beneficial biological effect of diet on the microbiome is attributed to its prebiotic components. In human breast milk, these components are linked to the carbohydrate fraction of the milk and referred to as Human Milk Oligosaccharides (HMOs). Their complex structure is based on lactose to which monosaccharides like fucose, N-acetylglucosamine and/or sialic acid are attached at specific linkage points. They are the third largest constituent of human milk after lactose and fat, and have been shown to selectively stimulate the growth of bifidobacteria & lactobacilli in the intestines [232425].
Currently there are more than 200 molecules of HMOs that have been characterized with the amount and composition varying substantially between lactating women and over the course of lactation [2627]. Different HMOs are likely to have different functions which presumably contribute to the variability in its composition; however prebiotics are also associated with other factors such as genetics, ethnicity, parity, geographic location, season of collection, and breastfeeding [28].
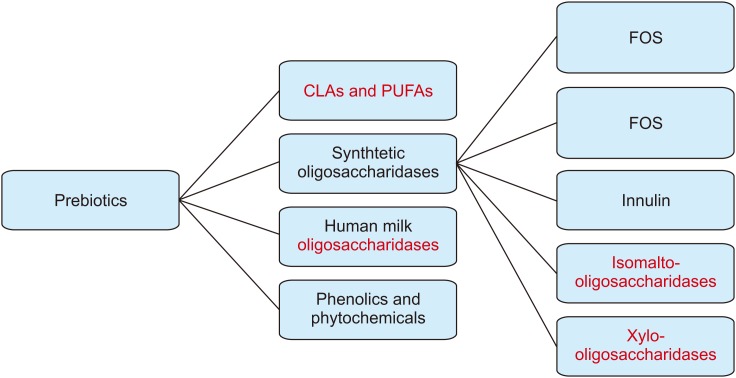
With the knowledge that bovine milk is almost completely devoid of milk oligosaccharides, recent biotechnical advances have made it possible to produce some synthetic milk oligosaccharides in large quantities. These advances enable supplementation of infant milk formula with the goal of promoting gut microbiota composition and function that is similar to that of a breast-fed infant [293031]. Preparations like galactooligosaccharides (GOS), fructooligosaccharides (FOS), 2'-fucosyllactose, lacto-N-neo-tetraose, inulin, oligofructose and galactofructose are examples of commonly used and studied products. Other sources of prebiotics include xylo-oligosaccharides, which are polymers of sugar xylose, produced from plant fiber, and isomalto-oligosaccharides, which are a mixture of digestion-resistant short chain carbohydrates naturally found in some foods as well as commercially manufactured products. Phytochemicals are a source of prebiotics and probiotics, and several chemical compounds such as polyphenols and derivatives, carotenoids and thiosulphates, which can promote gut microbiota function and are therefore being explored as a treatment for obesity and inflammatory diseases in adults (Fig. 1) [32]. It is important to note that those added to infant formula are synthetic and not human, thus it is more accurate to refer to them as MO's ‘Milk oligosaccharides’ rather than HMO's ‘HMOS’, as such inaccurate labelling may be deceiving to consumers. Although, these synthetic oligosaccharides are still not fully similar in structure to natural HMOs, their addition to milk formulas administered to healthy infants is thought to have potential positive effects and does not raise safety concerns with regards to infant development or result in other adverse effects [3334]. Infant formulae supplemented with GOS and FOS are well-studied in randomized clinical studies with proven benefits in terms of reducing infections and risk of allergy [32].
Go to : 
The definition of prebiotics has evolved significantly over the last two decades. The concept of prebiotics was first introduced in 1995 as “a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health [35].” Only substances that affect a limited number of bacteria in the gut, namely that of bifidobacteria and lactobacilli, were considered in early discussions. In 2004, the definition of prebiotic was updated to “selectively fermented ingredients that allow specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health”, therefore imposing the condition that claimed beneficial effects should be proven in the target host. To do so, prebiotics should resist host digestion and enable fermentation by intestinal microbiota [36].
With advances in molecular methods and increasing evidence about the diversity and density of bacterial communities, The International Scientific Association for Probiotics and Prebiotics (ISAPP), in 2010, issued a consensus statement updating the definition of dietary prebiotic as “a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health [37].” This updated definition includes a non-specific number of bacterial species. It further expands the location considered, from only the colon to the entire length of the GI tract.
In 2015, Bindels and colleagues [38] proposed that a prebiotic should be defined as “a non-digestible compound that, through its metabolization by microorganisms in the gut, modulates the composition and/or activity of the gut microbiota, thus, conferring a beneficial physiological effect on the host.” Although this update eliminated microorganism specificity and selective fermentation processes as essential requirements, it still limited the prebiotics to interactions with gut microbiota excluding accordingly extra-intestinal habitats such as skin, respiratory tract, and vagina [38]. More recently, and armed with the latest scientific and clinical developments, ISAPP reconvened in December 2017 to expand the scope of the concept of prebiotic to “a substrate that is selectively utilized by host microorganisms conferring a health benefit.” Whilst retaining the microbiota-mediated health benefits, prebiotics accordingly, are not limited to food or carbohydrate substances only and are no longer restricted to the GI. They are now inclusive of non-food elements and applicable to extra-intestinal tissues. Furthermore, this definition is now applicable to animals [39].
Go to : 
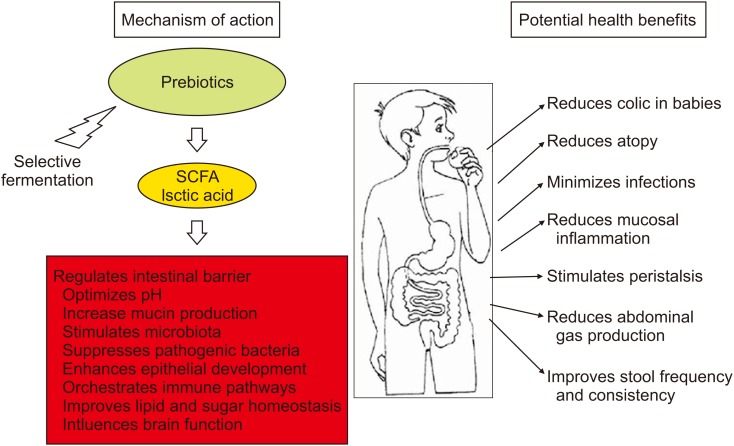
Despite the many advances in elucidating the mechanism of action of prebiotics, they remain partially elusive. The mechanism of action of prebiotics is postulated to be largely due to indirect effects. This includes acting as a fuel source for selective fermentation by resident health-promoting microorganisms of the GI tract, which are required for protecting against pathogens, or to improve intestinal barrier function, orchestrate immune pathways and influence brain function [4041]. Short chain fatty acids (SCFAs) are the main end products of selective fermentation. They mediate the direct effects of the prebiotics by providing an energy source to the gut epithelium. They also play a role in local gene expression by improving accessibility to transcription factors, enhancing intestinal barrier by regulating the assembly of tight junction proteins, improving gut motility, metabolite absorption, sugar and lipid homeostasis and immune function (Fig. 2). Acetate, propionate, and butyrate are the major SCFAs formed out of the fermentation process. Along with lactic acid, they participate in lowering the pH of the gut to levels that inhibit the growth of pathogens [42]. SCFAs are also thought to increase mucin production that can contribute to a lower incidence of bacterial translocation across the gut barrier [43]. Prebiotics, such as GOS, can exert a direct antimicrobial effect by adhering to the binding sites of bacteria on the enterocyte surface and thus, block the adhesion of pathogenic bacteria to intestinal epithelial cells [4445].
Go to : 
According to the systematic review by the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition published in the year 2011, the most commonly studied prebiotic was a 9:1 mixture of short-chain GOS (scGOS) and long-chain FOS (lcFOS); the predominant short chain to mimic the composition found in breast milk. Other prebiotics studied were GOS, acidic oligosaccharides (AOS), GOS/FOS/AOS, oligofructose plus inulin, and polydextrose plus GOS (with or without lactulose). Doses of various prebiotics in different studies ranged from 0.15 to 0.8 g/100 mL with a variable duration of intervention ranging from 2 weeks to 6 months [34]. Due to the limited numbers and heterogeneity of the various studies reviewed by the working group it was difficult to draw any robust conclusions based on these results. However, the potential beneficial effects of prebiotics were recognized; these effects included improvement in gut immunity, reduction in some atopic conditions, and alleviation of recurrent infections and inflammation. The use of prebiotics for functional diseases was also appreciated by the ESPGHAN working group and further appraised in a systematic review by the Dutch group in 2016 [34].
Go to : 
The GALT is the largest lymphoid tissue in the body, and mature lymphocytes in the gut mucosa vastly outnumber those in the bone marrow. A coordinated interaction between the gut microbiota and the immune system exists, allowing the host to tolerate the abundance of antigens present in the gut. Modulation of various GALT-associated immunological processes is thought to be the means through which prebiotics exert their beneficial effects. This is understood to be accomplished indirectly through increasing populations of beneficial microbes, especially lactic acid producing bacteria and bifidobacteria, in the gut. These probiotics, in turn increase the expression of anti-inflammatory cytokines, whilst reducing the expression of proinflammatory cytokines [46]. Butyrate, one of the SCFAs, was found to be associated with an increase in T-regulatory cells and a reduction in the production of IFN-γ. These findings, in conjunction with its effects on colonic epithelial proliferation and barrier function, suggests that butyrate is an important negative regulator of inflammation [47]. Moreover, acetate, the most abundantly produced SCFA in the colon, has been shown to exert anti-inflammatory effects through specific receptors that are expressed in adipose tissue and peripheral blood cells. Its high concentration in the bloodstream lead to the belief that systemic anti-inflammatory effects of this SCFA might be observed in other auto-immune diseases [4849].
The correlation between Bifidobacterium and the amount of intestinal secretory IgA has been well established [5051]. Although the addition of a specific mixture of 0.6 g/100 mL of a GOS and FOS in a 9:1 ratio to infant formula showed that intestinal secretory IgA concentration at weeks 8 and 26 did not differ from the control group, after 26 weeks of the intervention there was a significant difference noted that was comparable to the breast-fed group [5253].
Go to : 
Increasing evidence suggests that the gut microbiome contributes to the pathophysiology of such inflammatory disorders [545556]. Research studying the differences in gut microbiota of atopic and non-atopic infants is of current interest [5758]. Kalliomäki et al. [57] investigated whether a specific composition of the early gut microflora precedes the later development of atopic sensitization. They analyzed the intestinal flora of infants at high risk of atopy at 3 weeks and 3 months of life. The infants were classified as atopic if they had at least one positive skin prick test at the age of 12 months. The results demonstrated that infants who exhibited atopy at the age of 12 months had more clostridia and fewer bifidobacteria in their stools at the age of three weeks than the non-atopic infants. Accordingly, it was suggested that preponderance of bifidobacteria was associated with maturation in immune function towards a non-atopic state [57].
The supplementation of prebiotics has been proposed as a possible method of intervention in preventing allergic disorders [5960]. In infants that are not exclusively breast-fed, evidence-based recommendations from the World Allergy Organization guideline panel and ESPGHAN Committee on Nutrition suggest the use of prebiotic supplementation as a preventative intervention for allergies. However, the same does not apply to infants that are exclusively breast-fed since the breast milk already contains large amount of prebiotic in addition to other protective components [345961].
One study conducted a small placebo-controlled investigation on the efficacy of prebiotic use of FOS in the treatment of eczema, which was assessed using the scoring atopic dermatitis index. The authors in this study reported significantly lower median scores in eczema compared to the placebo group after treatment for 6 weeks and 12 weeks [60].
Another dietary intervention study conducted by Arslanoglu et al. [62], determined that in the first two years of life, supplementation of a prebiotic mixture (8 g/L of scGOS/lcFOS) results in significantly reduced incidences of allergic manifestations, such as recurrent wheezing, atopic dermatitis and allergic urticaria. These effects were noted to last even after the completion of the intervention; in other words, this suggests a long-lasting immune modulating effect of the prebiotic mixture [62].
The majority of the meta-analysis and systematic reviews conducted in this area concluded that although the studies determine the use of prebiotics to positively impact allergic manifestations, the existing evidence is insufficient, and thus, further rigorous testing is required before prebiotics can be recommended as a routine method for allergy prevention in formula-fed infants [3463]. This is, in part, due to the many other possible factors that contribute to the development of allergies.
Go to : 
Supplementation of infant milk formula with a specific oligosaccharide composition (GOS/FOS) is shown in different experiments including randomized controlled trial (RCT) to significantly increase the number of bifidobacteria and reduce the number of pathogens such as E. coli, clostridia, and eubacteria in infants and older children when compared with a group of infants fed an un-supplemented formula [6465]. Furthermore, they result in stool characteristics that are similar to those found in infants fed human milk, suggesting a better gastrointestinal tolerance [66]. Such effects, though, can be dose dependent, with better results being observed following increased dosages [6768]. However, the clinical relevance of these observations remains questionable and unclear [34].
Oligosaccharide prebiotics were also found in RCT over a 6-month period to significantly reduce the number of infectious episodes (gastrointestinal and respiratory infections) and the incidence of recurring, particularly respiratory, infections during the first 6 months of life. It was postulated that immune modulating effects of the prebiotic mixture through alterations in the intestinal flora is the principal factor driving the observed preventative mechanism early in life [69].
Go to : 
Prebiotics enables correction of potential environmental triggers such as the dysbiosis between disease-inducing and protective intestinal flora that induces and perpetuates chronic inflammation of the bowel and other extra-intestinal organs. By selectively stimulating the growth of protective microorganisms such as Bifidobacterium and enhancing the resistance to colonization with disease-inducing bacteria like that of Bacteroides spp., prebiotics have been shown to contribute to the reduction of inflammation [7071]. Some prebiotics were found to be beneficial in certain animal models of colitis. In humans, given on its own or in combination with probiotics, prebiotics resulted in some improvement in some parameters of inflammatory bowel disease (IBD) in a few small, controlled studies. Though promising, the number of patients recruited to these studies was too small to draw meaningful conclusions [72]. The above findings are nonetheless very informative and remain the focus for further better designed studies on the use of prebiotics in IBD.
Go to : 
There have been significant advances in the definition and classification of functional gastrointestinal disorders in children, however the etiology remains unclear. Infantile colic is a fairly common functional disorder among young infants but remains largely misunderstood. Recently, disturbances in the gut microbiota have been implicated in the causation of colic through its effect on gut motility which can impact on gaseous production and therefore excessive crying. There is evolving data specifically showing that there is less diverse microbiota and lesser numbers of bifidobacteria and lactobacilli in infants with colic compared to controls [7374].
In an observational study on 214 infants with colic aged up to 3 months, Savino et al. [75], showed that the frequency of colic was reduced in 79% of infants who received a formula containing 90% ScGOS, 10% Lc-FOS, sn-2 palmitic acid and partially hydrolyzed proteins. The majority of related studies indicated that when taken in sufficient amounts, prebiotics soften stools, increase stool frequency without diarrhea, and increase the ratio of bifidobacteria to total fecal bacteria [7677].
In an RCT, using inulin-type fructans (containing 70% oligofructose, 30% IcFOS) supplementation significantly improved stool consistency, frequency, and texture to a softer consistency over time [78]. This finding was consistent with those of previous studies conducted on infants and adults and suggests that laxative use in treatment of constipation could potentially be reduced with the implementation of prebiotic treatment [798081]. However, a more recent systematic review failed to identify robust data to recommend using prebiotics for the treatment of constipation [82]. A plausible explanation for the lack of recommendation is that there are numerous other contributing causative factors for constipation.
With regard to irritable bowel syndrome and functional abdominal pain, there have been no published randomized controlled trials identified investigating the effect of prebiotics in children. There are two pediatric studies looking at the effect of low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols diet in children; however, both groups did not study the long-term beneficial effects and both lacked statistical validation, therefore it is difficult to draw any robust conclusions from these findings [82].
Go to : 
Prebiotics are now recognized as a promising therapeutic tool in the prevention and treatment for numerous disease states in children and for promoting overall health. Indeed, considering the extremely low risk of serious adverse effects, ease of administration, and strong potential to influence the composition and function of the microbiota in the gut and beyond, the beneficial clinical application of prebiotics seems promising. Prebiotics, are emerging as an immunoactive ingredient that may possess the potential of exerting long-lasting effects. This concept will further evolve to encompass future novel health potentials applicable to any microbial community to achieve advantageous effects beyond the food and pharmaceutical domains making. As technology advances, the development of curated prebiotic molecules with specific functional properties is an attractive and potential future achievement. Along with the calls for their free use, there remains a magnitude of unanswered questions related to their clinical significance, efficacy, mechanism of action, and possible long-term side effects. This highlights the need for further well-conducted research on the diversity and clinical applicability of prebiotics in health and disease.
Go to : 
References
1. Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000; 106:935–937. PMID: 11032852.

3. Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008; 153(Suppl 1):3–6. PMID: 18721321.

4. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444:1027–1031. PMID: 17183312.

5. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011; 121:2126–2132. PMID: 21633181.

6. Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012; 9:599–608. PMID: 22907164.

7. Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006; 124:837–848. PMID: 16497592.

8. Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, Trancassini M, et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016; 39:1–12. PMID: 26922981.
9. Hansen R, Scott KP, Khan S, Martin JC, Berry SH, Stevenson M, et al. First-pass meconium samples from healthy term vaginally-delivered neonates: an analysis of the microbiota. PLoS One. 2015; 10:e0133320. PMID: 26218283.

10. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014; 6:237ra65.

11. Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017; 5:48. PMID: 28454555.

12. Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009; 98:229–238. PMID: 19143664.

13. Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008; 138:1796S–800S. PMID: 18716189.

14. Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999; 28:19–25. PMID: 9890463.
15. Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery - effects on gut microbiota and humoral immunity. Neonatology. 2008; 93:236–240. PMID: 18025796.

16. Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999; 69:1035S–1045S. PMID: 10232646.

17. Benno Y, Sawada K, Mitsuoka T. The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol Immunol. 1984; 28:975–986. PMID: 6513816.

18. Penders J, Stobberingh EE, Thijs C, Adams H, Vink C, van Ree R, et al. Molecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developing. Clin Exp Allergy. 2006; 36:1602–1608. PMID: 17177684.

19. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000; 30:61–67. PMID: 10630441.

20. Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002; 68:219–226. PMID: 11772630.

21. Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006; 118:511–521. PMID: 16882802.

22. Li M, Wang M, Donovan SM. Early development of the gut microbiome and immune-mediated childhood disorders. Semin Reprod Med. 2014; 32:74–86. PMID: 24390924.

23. Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006; 38(Suppl 2):S291–S294. PMID: 17259094.

24. Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000; 20:699–722. PMID: 10940350.

25. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015; 91:619–622. PMID: 26375354.

26. McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, et al. What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. 2017; 105:1086–1100. PMID: 28356278.

27. Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS One. 2011; 6:e17315. PMID: 21423604.

28. Miliku K, Robertson B, Sharma AK, Subbarao P, Becker AB, Mandhane PJ, et al. CHILD Study Investigators. Bode L, Azad MB. Human milk oligosaccharide profiles and food sensitization among infants in the CHILD Study. Allergy. 2018; 73:2070–2073. PMID: 29775217.
29. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010; 104(Suppl 2):S1–S63.

30. Stewart ML, Timm DA, Slavin JL. Fructooligosaccharides exhibit more rapid fermentation than long-chain inulin in an in vitro fermentation system. Nutr Res. 2008; 28:329–334. PMID: 19083428.

31. Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015; 32:42–46. PMID: 25448231.

32. Moro G, Boehm G. Clinical outcomes of prebiotic intervention trials during infancy: a review. Functional Food Rev. 2012; 4:101–113.
33. Newburg DS. Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr. 2000; 30(Suppl 2):S8–S17. PMID: 10749396.

34. Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, et al. ESPGHAN Committee on Nutrition. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011; 52:238–250. PMID: 21150647.

35. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125:1401–1412. PMID: 7782892.

36. Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004; 17:259–275. PMID: 19079930.

37. Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, et al. Dietary prebiotics: current status and new definition. Food Sci Tech Bull Funct Food. 2010; 7:1–19.

38. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015; 12:303–310. PMID: 25824997.

39. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017; 14:491–502. PMID: 28611480.

40. Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012; 107:601–613. PMID: 21767445.

41. Wasilewski A, Zielińska M, Storr M, Fichna J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:1674–1682. PMID: 25822014.

42. Jakobsdottir G, Nyman M, Fåk F. Designing future prebiotic fiber to target metabolic syndrome. Nutrition. 2014; 30:497–502. PMID: 24262515.

43. Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000; 46:218–224. PMID: 10644316.

44. Gibson GR, McCartney AL, Rastall RA. Prebiotics and resistance to gastrointestinal infections. Br J Nutr. 2005; 93(Suppl 1):S31–S34. PMID: 15877892.

45. Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. 2006; 74:6920–6928. PMID: 16982832.
46. Shokryazdan P, Faseleh Jahromi M, Navidshad B, Liang JB. Effects of prebiotics on immune system and cytokine expression. Med Microbiol Immunol. 2017; 206:1–9. PMID: 27704207.

47. Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res. 2003; 1:855–862. PMID: 14517348.
48. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009; 461:1282–1286. PMID: 19865172.

49. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013; 341:569–573. PMID: 23828891.
50. Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998; 42:39–44. PMID: 9706796.

51. Yasui H, Mike A, Ohwaki M. Immunogenicity of Bifidobacterium breve and change in antibody production in Peyer's patches after oral administration. J Dairy Sci. 1989; 72:30–35. PMID: 2925954.

52. Raes M, Scholtens PA, Alliet P, Hensen K, Jongen H, Boehm G, et al. Exploration of basal immune parameters in healthy infants receiving an infant milk formula supplemented with prebiotics. Pediatr Allergy Immunol. 2010; 21(2 Pt 2):e377–e385. PMID: 20003064.

53. Ben XM, Zhou XY, Zhao WH, Yu WL, Pan W, Zhang WL, et al. Supplementation of milk formula with galacto-oligosaccharides improves intestinal micro-flora and fermentation in term infants. Chin Med J (Engl). 2004; 117:927–931. PMID: 15198901.
54. Muir AB, Benitez AJ, Dods K, Spergel JM, Fillon SA. Microbiome and its impact on gastrointestinal atopy. Allergy. 2016; 71:1256–1263. PMID: 27240281.

55. Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients. 2017; 9:E672. PMID: 28657607.

56. West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, et al. in-FLAME Microbiome Interest Group. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015; 135:3–13. quiz 14. PMID: 25567038.

57. Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001; 107:129–134. PMID: 11150002.

58. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018; 141:e20172437. PMID: 29519955.

59. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010; 22:626–634. PMID: 20733491.

60. Shibata R, Kimura M, Takahashi H, Mikami K, Aiba Y, Takeda H, et al. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin Exp Allergy. 2009; 39:1397–1403. PMID: 19508323.

61. Cuello-Garcia CA, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Morgano GP, Zhang Y, et al. World Allergy Organization-Mcmaster University guidelines for allergic disease prevention (GLAD-P): prebiotics. World Allergy Organ J. 2016; 9:10. PMID: 26962387.

62. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008; 138:1091–1095. PMID: 18492839.

63. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013; (3):CD006474. PMID: 23543544.

64. Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, et al. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005; 40:36–42. PMID: 15625424.

65. Boehm G, Jelinek J, Stahl B, van Laere K, Knol J, Fanaro S, et al. Prebiotics in infant formulas. J Clin Gastroenterol. 2004; 38(6 Suppl):S76–S79. PMID: 15220664.

66. Boehm G, Lidestri M, Casetta P, Jelinek J, Negretti F, Stahl B, et al. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002; 86:F178–F181. PMID: 11978748.

67. Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, et al. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr. 2002; 34:291–295. PMID: 11964956.

68. Moreno Villares JM. [Probiotics in infant formulae. Could we modify the immune response?]. An Pediatr (Barc). 2008; 68:286–294. Spanish. PMID: 18358143.
69. Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007; 137:2420–2424. PMID: 17951479.

70. Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999; 129(7 Suppl):1438S–1441S. PMID: 10395616.

71. Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004; 126:1620–1633. PMID: 15168372.

72. Looijer-van Langen MA, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. 2009; 15:454–462. PMID: 18831524.
73. de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. 2013; 131:e550–8. PMID: 23319531.

74. Dubois NE, Gregory KE. Characterizing the intestinal microbiome in infantile colic: findings based on an integrative review of the literature. Biol Res Nurs. 2016; 18:307–315. PMID: 26721871.
75. Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. “Minor” feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr Suppl. 2003; 91:86–90. PMID: 14599049.

76. Sherman PM, Cabana M, Gibson GR, Koletzko BV, Neu J, Veereman-Wauters G, et al. Potential roles and clinical utility of prebiotics in newborns, infants, and children: proceedings from a global prebiotic summit meeting, New York City, June 27-28, 2008. J Pediatr. 2009; 155:S61–S70. PMID: 19840609.

77. Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Böckler HM, et al. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003; 36:343–351. PMID: 12604972.
78. Closa-Monasterolo R, Ferré N, Castillejo-DeVillasante G, Luque V, Gispert-Llaurado M, Zaragoza-Jordana M, et al. The use of inulin-type fructans improves stool consistency in constipated children. A randomised clinical trial: pilot study. Int J Food Sci Nutr. 2017; 68:587–594. PMID: 27931142.

79. Ziegler E, Vanderhoof JA, Petschow B, Mitmesser SH, Stolz SI, Harris CL, et al. Term infants fed formula supplemented with selected blends of prebiotics grow normally and have soft stools similar to those reported for breast-fed infants. J Pediatr Gastroenterol Nutr. 2007; 44:359–364. PMID: 17325558.

80. Fanaro S, Marten B, Bagna R, Vigi V, Fabris C, Peña-Quintana L, et al. Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 2009; 48:82–88. PMID: 19172129.

81. Sabater-Molina M, Larqué E, Torrella F, Zamora S. Dietary fructooligosaccharides and potential benefits on health. J Physiol Biochem. 2009; 65:315–328. PMID: 20119826.

82. Koppen IJ, Benninga MA, Tabbers MM. Is there a role for pre-, pro- and synbiotics in the treatment of functional constipation in children? A systematic review. J Pediatr Gastroenterol Nutr. 2016; 63(Suppl 1):S27–S35. PMID: 27380596.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download