1. Kang KS. Nutritional counseling for obese children with obesity-related metabolic abnormalities in Korea. Pediatr Gastroenterol Hepatol Nutr. 2017; 20:71–78. PMID:
28730130.

2. Oh MS, Kim S, Jang JH, Park JY, Kang HS, Lee MS, et al. Associations among the degree of nonalcoholic fatty liver disease, metabolic syndrome, degree of obesity in children, and parental obesity. Pediatr Gastroenterol Hepatol Nutr. 2016; 19:199–206. PMID:
27738602.

3. Grant-Guimaraes J, Feinstein R, Laber E, Kosoy J. Childhood overweight and obesity. Gastroenterol Clin North Am. 2016; 45:715–728. PMID:
27837784.

4. Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013; 106:135–141. PMID:
23428692.

5. Johnson W, Stovitz SD, Choh AC, Czerwinski SA, Towne B, Demerath EW. Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int J Obes. 2012; 36:535–541.

6. Stovitz SD, Demerath EW, Hannan PJ, Lytle LA, Himes JH. Growing into obesity: patterns of height growth in those who become normal weight, overweight, or obese as young adults. Am J Hum Biol. 2011; 23:635–641. PMID:
21630370.

7. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51:1–25.

8. Chu MA, Choe BH. Obesity and metabolic syndrome among children and adolescents in Korea. J Korean Med Assoc. 2010; 53:142–152.

9. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:745–750. PMID:
12198701.

10. Lambert M, Paradis G, O'Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int J Obes. 2004; 28:833–841.

11. Cho KY, Park H, Seo JW. The relationship between lifestyle and metabolic syndrome in obese children and adolescents. Korean J Pediatr Gastroenterol Nutr. 2008; 11:150–159.

12. Tanner JM, Healy MJR, Goldstein H, Cameron N. Assessment of skeletal maturity and prediction of adult height (TW3 method). London: W.B: Saunders;2001.
13. Klein KO, Newfield RS, Hassink SG. Bone maturation along the spectrum from normal weight to obesity: a complex interplay of sex, growth factors and weight gain. J Pediatr Endocrinol Metab. 2016; 29:311–318. PMID:
26565541.

14. Sopher AB, Jean AM, Zwany SK, Winston DM, Pomeranz CB, Bell JJ, et al. Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity (Silver Spring). 2011; 19:1259–1264. PMID:
21311512.

15. Lee HS, Shim YS, Jeong HR, Kwon EB, Hwang JS. The association between bone age advancement and insulin resistance in prepubertal obese children. Exp Clin Endocrinol Diabetes. 2015; 123:604–607. PMID:
26600056.

16. Gurnurkar S, Arheart KL, Messiah SE, Mankodi A, Carrillo A. Skeletal maturation and predicted adult height in children with premature adrenarche. J Pediatr Endocrinol Metab. 2014; 27:69–74. PMID:
23959660.

17. Corvalán C, Uauy R, Mericq V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am J Clin Nutr. 2013; 97:318–325. PMID:
23283497.

18. Keane VA. Assessment of growth. In : Kliegman RM, Stanton BF, St. Geme III, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia: Elsevier;2016.
19. Baker S, Barlow S, Cochran W, Fuchs G, Klish W, Krebs N, et al. Overweight children and adolescents: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005; 40:533–543. PMID:
15861011.

20. He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001; 49:244–251. PMID:
11158521.

21. Russell DL, Keil MF, Bonat SH, Uwaifo GI, Nicholson JC, McDuffie JR, et al. The relation between skeletal maturation and adiposity in African American and Caucasian children. J Pediatr. 2001; 139:844–848. PMID:
11743511.

22. Wu S, Aguilar AL, Ostrow V, De Luca F. Insulin resistance secondary to a high-fat diet stimulates longitudinal bone growth and growth plate chondrogenesis in mice. Endocrinology. 2011; 152:468–475. PMID:
21106874.

23. Pinhas-Hamiel O, Benary D, Mazor-Aronovich K, Ben-Ami M, Levy-Shraga Y, Boyko V, et al. Advanced bone age and hyperinsulinemia in overweight and obese children. Endocr Pract. 2014; 20:62–67. PMID:
24013996.

24. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017; 64:319–334. PMID:
28107283.

25. Pludowski P, Litwin M, Niemirska A, Jaworski M, Sladowska J, Kryskiewicz E, et al. Accelarated skeletal maturation in children with primary hypertension. Hypertension. 2009; 54:1234–1239. PMID:
19841285.

26. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010; 140:399–410. PMID:
20802107.

27. De Leonibus C, Marcovecchio ML, Chiarelli F. Update on statural growth and pubertal development in obese children. Pediatr Rep. 2012; 4:e35. PMID:
23355935.

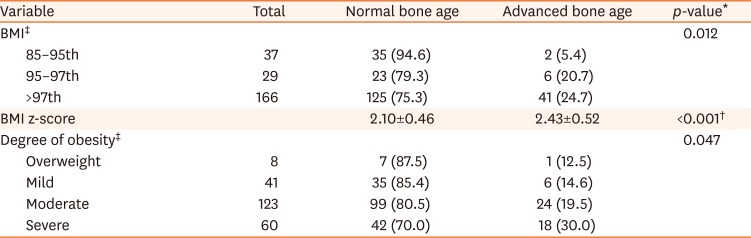
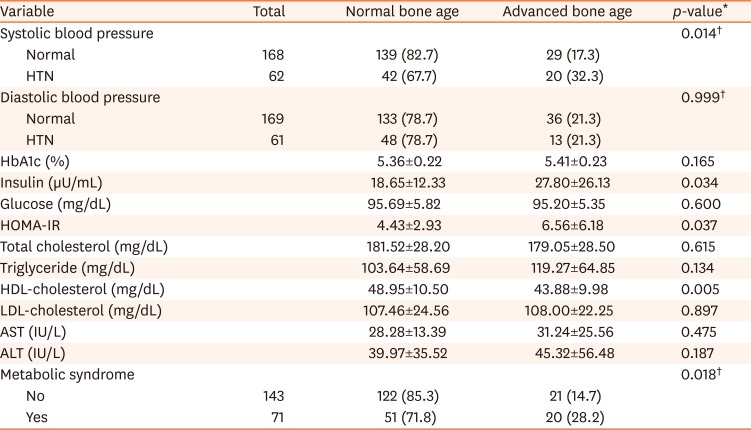
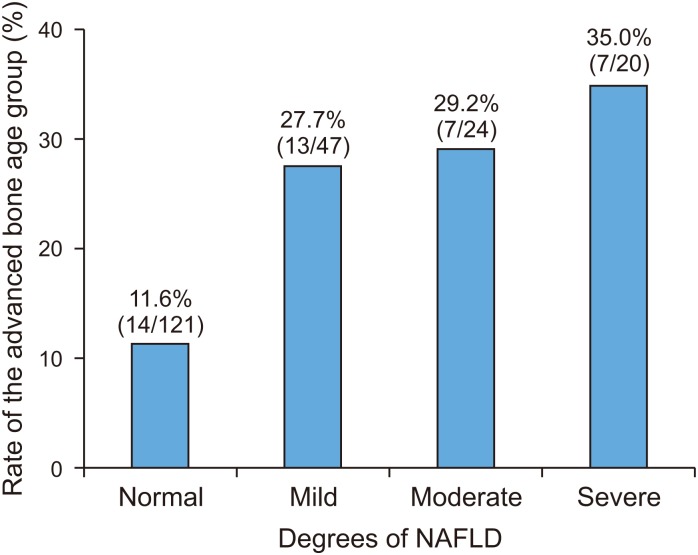
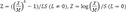 [7]. The degree of obesity was defined as the percentage of the actual weight relative to the standard weight for that height by sex and divided into grades: overweight (10–20%), mild (20–30%), moderate (30–50%), and severe (≥50%) [8]. We classified the anthropometric data into each section according to the percentile, and divided children into the normal or advanced bone age groups in each percentile section. We analyzed the trends of the prevalence of advanced bone age according to the change in each percentile section.
[7]. The degree of obesity was defined as the percentage of the actual weight relative to the standard weight for that height by sex and divided into grades: overweight (10–20%), mild (20–30%), moderate (30–50%), and severe (≥50%) [8]. We classified the anthropometric data into each section according to the percentile, and divided children into the normal or advanced bone age groups in each percentile section. We analyzed the trends of the prevalence of advanced bone age according to the change in each percentile section.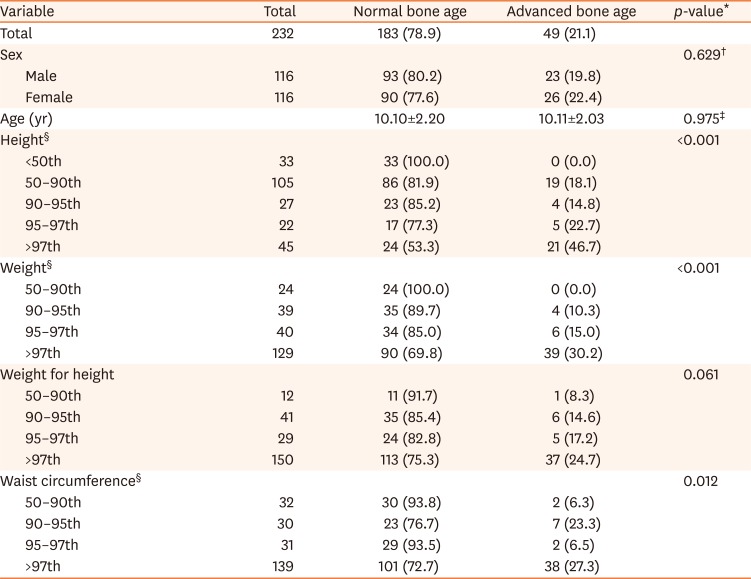




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download