INTRODUCTION
Intracranial surgeries are becoming more common in small animals, however literature on anesthetic management is very limited [
1]. Anesthetic management should optimize cerebral perfusion and oxygenation, avoiding hypo- or hypertension and promoting a rapid, but smooth, recovery.
Propofol or sevoflurane, both combined with an opioid, are commonly used to anesthetize humans undergoing intracranial surgery with similar outcome [
2]. Dexmedetomidine is gaining popularity because it facilitates endotracheal intubation with minimal ventilatory depression, maintains arterial blood pressure, and decreases opioids and anesthetic agents requirement [
3]. Additionally, dexmedetomidine may reduce cognitive dysfunction related to ischemic cerebrovascular disease [
4]. In dogs, dexmedetomidine inhibits isoflurane and sevoflurane induced cerebral vasodilation [
5], which is advantageous in controlling intracranial pressure (ICP). Additionally, its perioperative use to smooth recovery and improve intra- and post-operative conditions has been reported in a dog [
6].
The main aim of this retrospective observational study is to report our clinical experience on using sevoflurane combined with either a short acting opioid or dexmedetomidine infusion to anesthetize dogs undergoing elective rostrotentorial or transfrontal craniectomy, comparing their effect on selected intra- and post-operative variables. The prevalence of post-operative complications was also considered. We hypothesized that both techniques would provide similar intra- and post-operative conditions for the variables studied.
MATERIALS AND METHODS
Due to the retrospective nature of the study, and because sensitive information was not used, ethical review was not pursued. The standard consent form used at Dick White Referrals includes request to consent for use of clinical data for publication.
Clinical records of dogs undergoing elective rostrotentorial or transfrontal craniectomy for brain tumor excision from March 2013 to May 2018 were retrieved querying the practice management system and the electronic theater log. Inclusion criteria were: tumor location in frontal or olfactory lobes, anesthetic maintenance with sevoflurane and either a short acting opioid or dexmedetomidine infusion. Records were excluded if information for more than one variable were incomplete, if the tumor was in a different location, if the anesthetic technique differed from the protocols considered, or if the owners had not consented to use of data.
Clinical records were reviewed and cases allocated to two groups according to the anesthetic technique used: 1) Sevo-Op, anesthesia was maintained with sevoflurane (SevoFlo; Zoetis, UK) combined with an opioid infusion; 2) Sevo-Dex, anesthesia was maintained with sevoflurane and dexmedetomidine (Dexdomitor; Vetoquinol, UK) infusion.
Variables listed in
Tables 1 and
2 were retrieved and transcribed on a excel spreadsheet. Bradycardia was defined as heart rate (HR) < 50 beat/min; tachycardia as HR > 120 beat/min; hypotension as invasive mean arterial pressure (MAP) < 70 mmHg for more than 15 min; hypertension as invasive MAP > 140 mmHg for more than 15 min. Hypothermia and hyperthermia were defined as esophageal temperature < 37.5°C and > 39°C, respectively. Average invasive MAP, HR, end-tidal partial pressure of CO
2 (P
E′CO
2), end-expiratory fraction of sevoflurane (F
E′Sevo), and opioid or dexmedetomidine infusion rate during surgery were calculated by averaging the dose over the duration of administration. The same multi-parameter monitor (Mindray Beneview T5, China) was used in the period examined, and information was retrieved from paper anesthetic records, in which variables were documented every five minutes. Medications administered intra-operatively were copied to the spreadsheet. Time between sevoflurane discontinuation and endotracheal extubation was calculated.
Table 1
Demographic data, ASA physical status, tumor location, surgical approach and pre-anesthetic therapy in dogs that underwent elective rostrotentorial or transfrontal craniectomy

|
Information recorded |
Sevo-Op |
Sevo-Dex |
P value |
|
No. of animals |
8 |
13 |
- |
|
Age (yr) |
9.64 (± 2.56) |
10.22 (± 1.85) |
0.59 |
|
Sex (No.) |
5F, 3M |
6F, 7M |
0.66 |
|
Breed (No.) |
1 Crossbreed; 1 French Bulldog; 1 German Shepherd; 1 Golden Retriever; 2 Labrador Retriever; 1 Springer Spaniel; 1 Staffordshire Bull Terrier |
2 Crossbreed; 1 Dobermann; 1 German Shepherd; 1 Labrador Retriever; 1 Newfoundland; 1 Poodle; 1 Springer Spaniel; 1 Stabyhoun; 1 Staffordshire Bull Terrier; 1 Weimaraner; 2 West Highland White Terrier |
N/A |
|
ASA status (No.) |
2 ASA-2, 6 ASA-3 |
3 ASA-2, 10 ASA-3 |
0.99 |
|
Tumor location (No.) |
5 Frontal |
5 Frontal |
0.38 |
|
3 Olfactory |
6 Olfactory |
|
2 Olfactory + Frontal |
|
Intra vs. extra-axial (No.) |
1 Intra-axial |
2 Intra-axial |
0.99 |
|
7 Extra-axial |
11 Extra-axial |
|
Surgical approach (No.) |
2 Rostrotentorial |
2 Rostrotentorial |
0.62 |
|
6 Transfrontal |
11 Transfrontal |
|
Pre-anesthetic therapy (No. [dose in mg/kg]) |
7 Mannitol (0.5) |
9 Mannitol (0.5) |
0.61 |
|
7 Phenobarbital (2.5 [1.6–2.9]) |
12 Phenobarbital (2.5 [1.5–3]) |
0.99 |
|
8 Dexamethasone (0.2 [0.05–0.2]) |
11 Dexamethasone (0.2 [0.2–0.4]) |
0.50 |
Table 2
Drugs and dosages [median (range)] administered during anesthesia, selected intra-operative cardio-respiratory variables (mean ± SD), number of intraoperative complications and time to tracheal extubation (mean ± SD)

|
Information recorded |
Sevo-Op |
Sevo-Dex |
P value |
Sevo-Op vs. Sevo-Dex |
|
OR (95% CI) |
p value |
|
Pre-anesthetic medication (No.) |
4 Methadone |
3 Methadone |
0.35 |
- |
- |
|
4 Methadone + Dexmedetomidine |
10 Methadone + Dexmedetomidine |
|
Methadone dose (mg/kg) |
0.22 ± 0.04 |
0.21 ± 0.03 |
0.41 |
- |
- |
|
Dexmedetomidine dose (µg/kg) |
0.65 ± 0.25 |
0.96 ± 0.48 |
0.14 |
- |
- |
|
Route of administration (No.) |
8 Intravenous |
10 Intravenous |
0.26 |
- |
- |
|
3 Intramuscular |
|
Induction of anesthesia (No.) & [(dose in mg/kg)] |
5 Propofol [2.6 (2–3.4)] |
9 Propofol [2.2 (1.1–2.9)] |
0.66 |
- |
- |
|
1 Propofol (3.1) + Lidocaine (1) |
2 Propofol (1.8) + Lidocaine (1) |
|
1 Alfaxalone (2) |
1 Alfaxalone (1.1) |
|
1 Alfaxalone (1.4) + Lidocaine (1) + Remifentanil (0.002) |
1 Alfaxalone (1) + Dexmedetomidine (0.0005) |
|
Intraoperative medications (No.) & [(dose in mg/kg)] |
8 Dexamethasone [0.2 (0.1–0.2)] |
5 Dexamethasone [0.2 (0.05–0.2)] |
0.007 |
- |
- |
|
1 Phenobarbital (2.5) |
1 Phenobarbital (2.9) |
0.99 |
|
1 Glycopirrolate (0.0075) |
2 Glycopirrolate (0.005–0.009) |
0.61 |
|
0 Labetalol |
1 Labetalol (2) mg/kg/h |
0.99 |
|
1 Acepromazine (0.01) |
2 Acepromazine (0.009–0.02) |
0.99 |
|
0 Paracetamol |
3 Paracetamol (10) |
0.99 |
|
|
0.26 |
|
Intraoperative infusion rates (No.) & [(µg/kg/min)] |
4 Remifentanil [0.25 (0.15–0.28)] |
13 Dexmedetomidine [0.012 (0.008–0.016)] |
N/A |
- |
- |
|
2 Alfentanil [0.62 (0.57–0.66)] |
1 Lidocaine (40) |
|
2 Fentanyl [0.085 (0.08–0.09)] |
|
|
1 Lidocaine (33) |
|
|
Heart rate (beats/min) |
62 ± 15 |
65 ± 22 |
0.79 |
- |
- |
|
Mean arterial pressure (mmHg) |
85 ± 7 |
98 ± 8 |
0.0009 |
- |
- |
|
PE′CO2 (mmHg) |
36 ± 3 |
38 ± 3 |
0.94 |
- |
- |
|
FE′Sevo (%) |
2.14 ± 0.21 |
2.11 ± 0.17 |
0.75 |
- |
- |
|
Intraoperative complications (No.) |
5 Bradycardia |
11 Bradycardia |
0.33 |
3.3 (0.41–26.37) |
0.26 |
|
0 Tachycardia |
1 Tachycardia |
0.99 |
2 (0.07–56.27) |
0.67 |
|
2 Hypotension |
1 Hypotension |
0.54 |
4 (0.30–53.47) |
0.29*
|
|
0 Hypertension |
3 Hypertension |
0.26 |
5.7 (0.26–125.56) |
0.27 |
|
0 AV block |
3 AV block |
0.26 |
5.7 (0.26–125.56) |
0.27 |
|
4 Hypothermia |
6 Hypothermia |
0.99 |
1.17 (0.2–6.80) |
0.86*
|
|
1 Hyperthermia |
3 Hyperthermia |
0.99 |
2.1 (0.18–24.6) |
0.55 |
|
Time to tracheal extubation (min) |
20 (10–25) |
38 (10–70) |
0.0033 |
- |
- |
Complications occurring during the first 24 post-operative hours were also recorded (
Table 3). Agitation was defined as presence of signs of disorientation and/or vocalization having ruled out pain (Glasgow Composite Pain Scale ≤ 5/20) [
7] and drug-induced dysphoria (behavioral changes observed after drug administration). Dexmedetomidine used as treatment for agitation (as described above) was considered preventive or reactive depending on whether it was administered in absence or presence of documented signs of agitation. Acepromazine was also used for the treatment of agitation, but in this case its administration was always preventive intra-operatively, and reactive post-operatively. In some instances, acepromazine was also used as a reactive first line treatment for hypertension. Labetalol was always administered as a treatment for hypertension (trigger values being as above).
Table 3
Number of post-operative complications, n. and doses of post-operative administration of labetalol, dexmedetomidine and acepromazine

|
Information recorded |
Sevo-Op |
Sevo-Dex |
p value |
Sevo-Op vs. Sevo-Dex |
|
OR (95% CI) |
p value |
|
Postoperative complications (No.) |
4 Bradycardia |
10 Bradycardia |
0.35 |
3.33 (0.50–22.14) |
0.21 |
|
1 Tachycardia |
2 Tachycardia |
0.99 |
1.27 (0.096–16.81) |
0.85 |
|
3 Hypertension |
8 Hypertension |
0.39 |
2.67 (0.43–16.40) |
0.29 |
|
4 Agitation |
8 Agitation |
0.67 |
1.6 (0.27–9.49) |
0.60 |
|
0 Seizures |
1 Seizures |
0.99 |
2.0 (0.074–56.27) |
0.67 |
|
2 Hypothermia |
8 Hypothermia |
0.18 |
4.8 (0.68–33.8) |
0.12 |
|
0 Hyperthermia |
2 Hyperthermia |
0.50 |
3.7 (0.16–87.39) |
0.42 |
|
Postoperative labetalol administration (No.) [(dose in mg/kg/h)] |
2 [1.75 (1.5–2)] |
6 [1.9 (1.3–2.4)] |
0.40 |
2.57 (0.37–17.83) |
0.34 |
|
Postoperative dexmedetomidine administration (No.) [(dose in µg/kg/min)] |
7 [0.015 (0.008–0.025)] |
13 [0.011 (0.005–0.023)] |
0.38 |
5.4 (0.19–149.79) |
0.32 |
|
Reason for postoperative dexmedetomidine (No.) |
6 Preemptive |
13 Preemptive |
0.35 |
- |
- |
|
1 Reactive |
0 Reactive |
|
Postoperative acepromazine administration (No.) [(dose in µg/kg)] |
5 [8.8 (5–10)] |
9 [10.4 (7–20)] |
0.99 |
1.35 (0.21–8.62) |
0.75 |
Continuous variables were all normally distributed (D'Agostino & Pearson normality test) and were analyzed with an unpaired
t-test with Welch's correction, reporting results as mean (± standard deviation). Categorical variables were compared using a Fisher's exact test. Statistical tests were performed using Prism 7 (GraphPad Prism 7 for Mac, GraphPad Software, USA,
www.graphpad.com).
Odds ratio (OR) and 95% Confidence Interval (CI) were calculated using Medcalc (Medcalc,
https://www.medcalc.org), for relevant categorical variables (e.g. likelihood of complications), adding 0.5 to all cells when a frequency was zero [
8]. Results were considered significant when
p values ≤ 0.05.
RESULTS
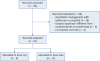
During the 5 years period examined 49 elective craniectomies were performed. Statistical analysis was performed on 21 records: 8 were assigned to the Sevo-Op group and 13 to the Sevo-Dex group; 28 records were excluded (
Fig. 1). Age, sex, American Society of Anesthesiologists status and tumor location were not significantly different between groups. Tumors were mostly extra-axial (
Table 1).
Fig. 1
Consolidated Standards of Reporting Trials diagram of the study.
Group Sevo-Op, sevoflurane and short acting opioid infusion; Group Sevo-Dex, sevoflurane and dexmedetomidine infusion.
No difference was found between groups for pre-operative use of mannitol, phenobarbital and dexamethasone (
Table 1). Drugs, doses and route of administration of pre-anesthetic medication administered were not significantly different between groups (
Table 2).
Anesthesia was induced using either propofol (PropoFlo; Abbott, UK) or alfaxalone (Alfaxan; Jurox, UK) with or without a co-induction agent (
Table 2). During surgery, alfentanil (Rapifen; Janssen-Cilag, UK), remifentanil (Ultiva; Aspen Pharma, Ireland) or fentanyl (Fentadon; Dechra, UK) were administered in Sevo-Op, while dexmedetomidine was administered in Sevo-Dex. One dog in each group also received lidocaine infusion (Lidocaine hydrochloride 2%; Hameln, UK). Infusion rates for each drug are presented in
Table 2. All opioid infusions were stopped before the end of the procedure, allowing sufficient time for their effect on ventilation to be minimal.
During surgery, all dogs received IV cefuroxime (20 mg/kg, Zinacef; GlaxoSmithKline, UK) after induction of anesthesia and then every 90 min throughout the procedure; mannitol (0.5 g/kg, Polyfusor Mannitol 10%; Fresenius Kabi, UK) IV over 20 min after induction of anesthesia, prior to starting surgery. Dexamethasone (Colvasone 0.2%; Norbrook, Northern Ireland) was administered to 8 and 5 dogs of group Sevo-Op and Sevo-Dex, respectively (
p = 0.0068) (
Table 2).
Sevoflurane was delivered in oxygen using a circle breathing system, and ventilation was controlled throughout the procedure (Draeger Fabius GS; Draeger, UK) in all animals using a volume-controlled modality. The mean P
E′CO
2 and F
E′Sevo were similar between groups (
Table 2). While intra-operative MAP was significantly higher in group Sevo-Dex (
p = 0.0009), HR was similar (
p = 0.79) (
Table 2). Bradycardia was the most common intra-operative complication (76% of cases). Overall, antimuscarinics were administered in three dogs for the following reasons: treatment of AV block (n = 1), treatment of sinus arrest (n = 1), improvement of MAP associated with inappropriately low HR (n = 1). The time to extubation was significantly longer in Sevo-Dex when compared to Sevo-Op (
Table 2).
The most common post-operative complications were bradycardia (66% of cases), agitation (57% of cases) and hypertension (52% of cases), with similar prevalence in both groups (
Table 2). The proportion of animals in which labetalol (Labetalol Hydrochloride; Focus, UK) or dexmedetomidine (either preemptively or reactively) were used to treat hypertension or sedate the animal, respectively, was similar between groups (
Table 2).
Acepromazine (ACP; Elanco, UK) was used intraoperatively in 3 out of 21 dogs. In one case it was given as preparation for recovery, while in the other two cases it was used to control hypertension, being efficacious in only one instance. Post-operatively, acepromazine was administered to 13 out of 21 dogs. In 11 dogs it was administered alongside dexmedetomidine infusion to provide sedation. In the other 2 dogs it was administered to control hypertension as a first option, but it was effective only in one case. When acepromazine was ineffective to decrease arterial blood pressure, labetalol was administered with success. All animals were discharged from the hospital.
DISCUSSION
This is the first study reporting and comparing the combination of sevoflurane with a short acting opioid or dexmedetomidine infusion, and the prevalence of intra- and post-operative complications in dogs undergoing elective intracranial surgery. Both intraoperative infusion of short acting opioids and dexmedetomidine as adjunct to sevoflurane allowed to achieve comparable anesthetic conditions. Both intra- and post-operative cardiovascular measures of outcome were similar between groups and this was not surprising, as drug administration was titrated to a similar clinical effect, and management of the case followed the guidelines commonly reported for this type of surgery to provide neuroprotection [
9].
In presence of a brain tumor, or suspected increased ICP, it has been suggested to maintain MAP between 80–100 mmHg to ensure adequate cerebral perfusion pressure (CPP) [
101112]. To reduce the amount of inhalational anesthetic agents needed to maintain general anesthesia, and minimize their negative effect on MAP, opioids are generally infused in dogs undergoing surgery [
13]. However, information about their administration during craniectomy are minimal and based on the only study published by Raisis et al. [
1] in which alfentanil was infused with propofol and a single case-report in which alfaxalone—remifentanil use is described [
14]. According to our results, fentanyl, alfentanil, remifentanil but also dexmedetomidine, can be combined with sevoflurane to maintain the MAP within the suggested range. The higher MAP detected in group Sevo-Dex is probably direct effect of the dose-related dexmedetomidine induced vasoconstriction, as reported in isoflurane-anesthetized dogs [
15]. Even if statistically significant, this result might not be clinically relevant, considering that the prevalence of intra-operative hypotension and hypertension was similar between groups and it should also be considered that MAP is an easily measurable but very crude measure of cerebral perfusion. To the best of our knowledge the intra-operative infusion of dexmedetomidine in dogs undergoing craniectomy has never been reported, therefore our results could be used as starting point for further research in the use of this drug in this specific population of animals. In humans undergoing craniectomy, dexmedetomidine is commonly infused to reduce occurrence of cough in response to intubation, decrease general anesthetic requirement, post-operative opioid consumption and cognitive dysfunction due to ischemic cerebrovascular disease [
34]. Further, the vasoconstrictive effect of dexmedetomidine on cerebral arterioles might be beneficial to counteract inhalational anesthetic-induced cerebral vasodilation [
5].
Intravenous anesthetics (i.e. propofol) reduce cerebral metabolism rate (CMR) and cerebral blood flow (CBF) without intrinsic cerebral vasodilatory effect in patients with intracranial diseases [
16]. Propofol infusion reduced the risk of hypotension and improved cardiac index in dogs with intracranial disease undergoing magnetic resonance imaging [
17], and its use has been described by Raisis et al. [
1] in dogs undergoing craniectomy. While the use of inhalational agents in this setting has been controversial, their net effect on CBF depends on the balance between CBF reduction caused by CMR suppression and CBF augmentation caused by direct cerebral vasodilation. At concentration < 1 minimum alveolar concentration (MAC), the effect of CMR suppression is predominant, but beyond 1 MAC the cerebral arteriolar vasodilatory effect might increase CBF and ICP [
18], leading to uncoupling between CBF and CMR. Using positron emission tomography, propofol decreased CBF in a dose-dependent manner without causing blood redistribution. At concentration < 1 MAC the effect of sevoflurane on CBF is similar to propofol, but at the doses > 1 MAC CBF is redistributed to the subcortex (i.e. cerebellum) [
19]. Similarly, cerebral autoregulation is preserved during administration of 1 MAC isoflurane, but it is not during administration of 2 MAC [
18]. For this reason, if either isoflurane or sevoflurane are used to maintain anesthesia in dogs with intracranial disease, the end-expiratory fraction should be limited at value < 1 MAC. In this study, the F
E′Sevo was similarly decreased infusing either opioids or dexmedetomidine, and the mean F
E′Sevo recorded was lower than the MAC of sevoflurane reported in dogs [
20]. However, due to the different MAC sparing effects of opioids and dexmedetomidine, we cannot be certain that all dogs were maintained at the same depth of anesthesia, and therefore this source of variability needs to be taken into account interpreting our findings. Furthermore, the retrospective nature of this study precluded to find the minimal F
E′Sevo necessary to maintain anesthesia in both groups; therefore, it is also possible that lower F
E′Sevo could have been administered.
Three different opioids (remifentanil, alfentanil or fentanyl) were infused in dogs of group Sevo-Op and this is a limitation of this small dataset. In humans undergoing craniotomy minimal differences were found comparing the three above mentioned opioids, which is not surprising, as provided their pharmacokinetics characteristics are taken into account when managing the infusion, the effect should be similar, considered they are all short acting mu agonists [
21]. The infusion rate used for alfentanil was similar (0.73 [0.25–1.5] µg/kg/min) to the one reported by Raisis et al. [
1] in dogs undergoing craniectomies. The lack of standardization was a source of variability and a limitation of our study, however this study does not only want to compare the effect of two drugs (opioid vs. alpha-2 agonist) but, considering the paucity of publications describing the anesthetic management of dogs undergoing craniectomy, to report our clinical experience.
The effect of CO
2 on CBF must be considered when anesthetizing dogs with intracranial disease: mild hypocapnia (34–36 mmHg) is used to control CBF and ICP [
22]. Hyperventilation decreased ICP and brain volume in human patients undergoing craniotomy, which in turn improved surgical conditions [
23]. While marked hypocapnia could have an overall negative effect on brain perfusion and can lead to cerebral ischemia [
1124], it could be argued that a lower P
E′CO
2 could have been maintained in dogs belonging to group Sevo-Dex. In practical terms, it is unlikely that the small and not significant difference observed could have any clinical relevance, particularly if the vasoconstrictive effect of dexmedetomidine and the low amount of inhalational anesthetic agent used are taken into account.
Bradycardia was the most frequent intraoperative complication, and this was not an unexpected finding as both opioids and dexmedetomidine are negative chronotropes [
1521]. Nevertheless, specific treatment with an antimuscarinic was deemed necessary in only 14% of cases (3 out of 21). Similar to our study, when propofol-alfentanil were used, the median HR ranged between 32–65 beats/min [
1].
The median extubation time recorded in the Sevo-Op group is similar to what has been reported by Raisis et al. [
1] after administration of propofol-alfentanil. The median extubation time in group Sevo-Dex was, however, considerably longer. Although dexmedetomidine infusions have been reported to shorten the time to extubation in humans when compared to midazolam [
25] and propofol [
26], we are not aware of any study in human or veterinary medicine comparing extubation time after dexmedetomidine or opioid infusion, used with sevoflurane. In Sevo-Dex group, dexmedetomidine infusion was started at the beginning of anesthesia and continued in recovery, while in Sevo-Op group the infusion was started at the end of the procedure, and opioid infusions were stopped before the end of the procedure to avoid ventilatory depression. It is plausible, but this is only a speculation, that since dexmedetomidine infusion was not stopped to allow a decrease in its plasma concentration, this resulted in a longer time to extubation. This is not necessarily a disadvantage, as infusion of dexmedetomidine is not associated with significant ventilatory depression at the doses used, therefore it may contribute to a smooth recovery allowing complete elimination of the inhalational anesthetic [
27], and blunting response to extubation [
28].
Bradycardia, hypertension and agitation were the most frequent post-operative complications recorded in both groups, and this is the first retrospective study documenting their incidence, albeit in a relatively small sample. Bradycardia can be consequent to drug administration (i.e. dexmedetomidine or opioids) or a baroreceptor reflex in presence of systemic or intracranial hypertension, as part of the response known as Cushing's triad. Agitation is commonly reported after brain surgery in humans and can increase the sympathetic outflow and trigger hypertension [
29]. For this reason, it was chosen to infuse dexmedetomidine post-operatively with the aim of either preventing or controlling agitation. However, the vasoconstriction caused by dexmedetomidine might have contributed to increasing arterial blood pressure, even if the doses used have been associated to minimal cardiovascular effects in dogs [
15]. Hypertension can be life-threatening and has been associated with an increased risk of intracranial hemorrhage in the early postoperative period in humans [
30]. Similar to our results, the incidence of post-craniotomy hypertension in humans ranges between 54%–91% [
30] and it is generally treated administering β-blockers (i.e. esmolol or labetalol) [
3132]. Labetalol, in particular, reduces arterial blood pressure blocking both alpha and beta adrenoceptors without affecting HR, ICP and CPP [
32]. Its efficacy in reducing arterial blood pressure has been previously reported only in experimental dogs [
3334]. Therefore, this is the first clinical report of the use of labetalol in a significant number of dogs, and based on our experience, it was effective in controlling hypertension where acepromazine failed to do so. The difference of incidence of post-operative hypothermia was not found significant between groups (2 in Sevo-Op vs. 8 in Sevo-Dex), however the numbers show a clear trend towards a higher incidence in Sevo-Dex. This lack of statistical significance may be a mere effect of the low number of cases enrolled and could potentially become significant with a larger sample size.
The retrospective nature of this study, and its possible effect on results, is its major limitation. The anesthetic techniques here described reflect the management of individual specialists or residents in anesthesia working in one referral center and were not standardized across the groups. Further, due to the small sample size, results should be interpreted with caution. Nevertheless, the two sevoflurane-based anesthetic techniques here described provided comparable conditions in dogs undergoing elective intracranial surgery. We decided to consider only animals undergoing elective craniectomy to remove frontal or olfactory lobe tumors to reduce variability, even if this meant decreasing significantly the number of animals included. Animals with neoplasia in a similar location are likely to show comparable clinical signs, and similar surgical approaches are used, with similar complications. The similar use of osmotherapy suggests that clinical signs of raised ICP were similar at presentation in the two groups. Further studies are also warranted to clarify the potential for dexmedetomidine to improve quality of recovery in this specific population of animals, given the important role this drug plays in postoperative period in humans undergoing similar procedures [
34].
In conclusion, this retrospective study suggests that the two sevoflurane-based anesthetic techniques provided comparable condition in dogs undergoing elective intracranial surgery of the frontal and olfactory lobes. We have reported, for the first time in veterinary medicine, the prevalence of the most common post-operative complications (hypertension, bradycardia and agitation) and their treatment. Animals in Sevo-Dex had a higher MAP during surgery, and this may be an aspect to consider when choosing the anesthetic protocol, based on individual risk factors and the desired outcome. Future randomized prospective studies are warranted to determine how the anesthetic technique can be improved and to provide better evidence in this field.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download