Abstract
A 12-year-old Warmblood mare was presented with an acute onset left hindlimb lameness associated with generalised soft tissue swelling of the entire limb and medial saphenous vein (MSV) thrombophlebitis. A presumptive diagnosis of extremity compartment syndrome (ECS) was made. Due to the clinical deterioration, emergency fasciotomy of the crural fascia and biopsy was performed. Histological and immunohistochemical examination of the samples confirmed a diagnosis of leiomyosarcoma likely originating from the tunica media of the MSV. This report is the first to describe an unique combination of ECS and thrombophlebitis associated with a leiomyosarcoma in a horse.
Leiomyosarcoma is a malignant neoplasm derived from the smooth muscle cells, which is particularly rare in horses. There are few reports in the veterinary literature of leiomyosarcomas affecting mainly the urogenital [1], gastrointestinal tract [2], and multicentric [3]. Other reports describe leiomyosarcoma as a solitary mass in the lungs [4], sinus [5], guttural pouch [6], and ocular annexes [7]. Only 1 case of leiomyosarcoma affecting the thigh region muscle has been reported [8]. To the author's knowledge, there are no reports of compartment syndrome caused by a neoplastic condition in the horse.
Extremity compartment syndrome (ECS) occurs when there is an increased interstitial pressure impeding tissue perfusion within an interfascial compartment [910]. More commonly reported causes of compartment syndrome are post-anaesthetic myopathy affecting mainly the gluteal and triceps muscles [1112]; intracompartment haemorrhage following a traumatic event has also been reported [13].
The aim of this case report is to describe a unique combination of ECS and thrombophlebitis associated with a leiomyosarcoma in a horse, likely originating from the tunica media of the medial saphenous vein (MSV).
A 12-year-old Dutch Warmblood show-jumping mare was referred to the clinic following a 10-day history of swelling of the left crural region, suspected to be the result of a kick injury. No lameness was initially observed by the owner during the first 7 days from the suspected accident and the swelling followed cycles of enlargement and spontaneous regression. On day 8, veterinary advice was sought due to acute lameness and worsening of the swelling. On physical examination, the referring veterinary surgeon reported the presence of marked oedema extended from the middle third of the crural region to the coronary band. The mare was 3/5 LH limb lame (American Association of Equine Practitioners [AAEP]) and resented palpation of the left superficial inguinal lymph nodes. Vital parameters were unremarkable.
Radiographic examination of the left crural and tarsal region was performed to rule out fracture. No bony abnormalities were detected but a soft tissue mass was identified. A presumptive diagnosis of lymphangitis/cellulitis was made by the referring veterinary surgeon, who initially administered systemic dexamethasone (Dexadreson; MSD Animal Health, UK) (0.01 mg/kg, intravenous injection [iv]). This resulted in partial resolution of the soft tissue swelling, which became consolidated and more localised at the level of the mid-crural region. Nevertheless, the LH limb lameness became more severe and was evident at walk (4/5 AAEP scale). Therefore, on day 10, the horse was referred to the clinic for further investigations.
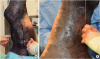
A localised, firm, moderately warm and painful swelling was present on the mid-third of the left crural region biaxially but more pronounced laterally (Fig. 1). The superficial veins were visible and appeared distended on the skin surface (Fig. 2). Palpable thickening of the MSV was detected along the entire course of the vein and the horse was very sensitive to palpation of the enlarged left superficial inguinal lymph nodes.
Haematology and biochemistry revealed only a mild hyperfibrinogenaemia (6.7 g/L, reference value 0.33–0.9 g/L). There were no abnormalities of the coagulation profile.
Ultrasonographic examination of the swelling revealed a soft tissue mass of mixed echogenicity with ill-defined margins situated caudal to the lateral digital flexor muscle. The normal muscular architecture was disrupted, especially on the medial aspect, and it was difficult to identify other anatomical structures. Thrombophlebitis of the MSV was detected and a thrombotic aneurysm (1.3 × 2 cm) was found on the cranial wall of the vein in the proximal crural region (Fig. 3C). Some portions of the vessel wall were not distinguishable from the surrounding tissue (Fig. 4). There was limited flow signal visible with colour Doppler (Fig. 3). Images were acquired using a portable ultrasound machine (Logiq E; GE, USA) with 5–13 MHz linear transducer. Transrectal ultrasonographic examination, using Esaote MyLabOneVET with 5–10 MHz linear rectal transducer, was performed to explore the extension of the thrombus and revealed a large (approximately 4 × 5 cm) mass with a mixed echogenicity (Fig. 5). This was suspected to be an enlarged sub-iliac lymph node.
A percutaneous fine needle aspirate was performed under ultrasonographic guidance on the lateral aspect of the swelling and submitted for bacteriologic and cytologic examination. The results were unhelpful. A presumptive diagnosis of ECS was made due to the panel of findings, which were deemed consistent with an organised hematoma and secondary MSV thrombophlebitis. Medical treatment included intramuscular procaine penicillin (Depocillin 300 mg/mL, MSD Animal Health) at 20 mg/kg, gentamicin (Genta-Equine 20 mg/mL; J.M. Loveridge, UK) at 6.6 mg/kg for 5 days and systemic anticoagulant therapy with acetylsalicylic acid (Aspirin; J.M. Loveridge) at 10 mg/kg per os. Phenylbutazone (Equipalazone 200 mg/mL; Dechra Veterinary Products, UK) (4.4 mg/kg iv), topical embrocation (Compagel; Boehringer Ingelheim Limited, UK) and hydrotherapy were maintained.
Due to the lack of improvement and deterioration of both the swelling and the lameness (5/5 AAEP scale), after eight days of hospitalization, emergency fasciotomy and open surgical biopsy were performed under general anesthesia in dorsal recumbency with the left hindlimb in extension.
Under ultrasonographic guidance, a 4 cm vertical incision was made on the caudo-lateral aspect of the crural region, over the lateral digital flexor approximately 10 cm proximal to the calcaneal tuberosity. A second, 3 cm, vertical incision was made 12 cm proximal to the first incision in the same plane. The fascia cruris (FC) was identified deep to the subcutaneous tissue, and was incised to allow the insertion of a fasciotome (Bathe-fasciotome; Dr Fritz Gmbh, Germany). The fasciotome was introduced parallel to the skin in a proximal direction and the FC of the lateral compartment was incised until the tip of the fasciotome was visible through the proximal incision (Fig. 6B).
During surgery it became apparent that the macroscopic findings were not consistent with a hematoma. The tissue appeared brown and parenchymatous in consistency. It was friable and easy to break down with gentle digital pressure. The region of the tibial caudalis muscle and lateral digital flexor consisted mostly of this abnormal tissue, which could not be effectively excised due to deep infiltration. The same surgical procedure was performed on the medial aspect, extending the incisions caudally to the thrombosed MSV under ultrasonographic guidance in order to avoid damage of the neurovascular structures. The tissue appeared similar to the tissue identified on the lateral aspect. Samples were collected and submitted for histopathologic examination.
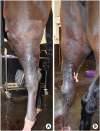
Skin incisions were left open and a Penrose drain was placed to permit drainage of the region (Fig. 7). Post-surgical treatment consisted in 10 minutes of in-hand walking exercise twice daily, hydrotherapy, continuation of systemic anticoagulant therapy with Aspirin (J.M. Loveridge) and analgesia (Phenylbutazone) (Equipalazone 200 mg/mL; Dechra Veterinary Products). The horse was significantly more comfortable in the post-operative period but the swelling remained.
The histopathological findings were consistent with the diagnosis of soft tissue sarcoma. The main differential diagnoses included fibrosarcoma, rhabdomyosarcoma, poorly differentiated leiomyosarcoma or, less likely, a tumour of perivascular wall origin. The histopathological examination confirmed the extensive nature of the lesion and based on margin assessment was deemed to be locally infiltrative. Further immunohistochemistry revealed positive staining for vimentin, desmin and smooth muscle actin and negative for cytokeratin. This supported a diagnosis of leiomyosarcoma.
The horse was euthanised at the owner's request due to the poor/hopeless prognosis. The owner did not consent to post-mortem examination.
Retrospectively, the MSV thrombophlebitis was thought to be a consequence of the voluminous swelling. As reported in the literature, an increased perivascular pressure can be responsible for impaired venous blood flow and therefore a cause of thrombotic processes [14].
The ultrasonographic examination was very similar to that described by Nelson et al. [13] in two horses diagnosed with antebrachial compartment syndrome, with ill-defined and diffuse areas with mixed echogenicity consistent with disruption of the normal muscular architecture. Due to the ultrasonographic similarity a presumptive diagnosis of ECS was made. ECS is well recognised in human medicine where it can be caused by tight bandages, trauma, snake venom, neoplasia, muscular exertion, vascular injury, haemophilia, and, most commonly, bone fracture [913]. In horses ECS is mainly attributed to post-anaesthetic myopathy and primarily affects the gluteal and triceps muscles [1112]. Nelson et al. [13] described compartment syndrome in the forelimb of two horses as a consequence of intra-compartment haemorrhage following a traumatic event. A similar aetiology was suggested by Bruniges et al. [10] in a case of ECS affecting the pelvic limb of a horse. The peculiarity of the presented case is that ECS appears to be secondary to the expansion of intracompartmental leyomiosarcoma coupled with a complex clinical picture, including a thrombophlebitis. Definitive diagnosis of compartment syndrome requires direct measurement of compartment pressure [10]. However, in equine medicine, this procedure is not normally available and therefore the diagnosis is reliant on history and clinical signs [10]. Furthermore, leiomyosarcoma in the horse is very rare. Only one case of leiomyosarcoma affecting the thigh region muscles has been reported [8], but this had a very different clinical presentation from the case reported here. This case had chronic, progressive clinical signs including weight loss and neurologic involvement, which was eventually attributed to the presence of cerebral metastases [8]. In the present case, metastases could not be confirmed due to the absence of post-mortem examination. However, due to the marked infiltrative nature of the tumour detected during the surgical exploration, it seems likely that this tumour at least had metastatic potential. The prognosis for future survival was considered poor. Furthermore, the owner of the horse declined further treatment and requested the horse to be euthanised. In human medicine, leiomyosarcoma has been classified into four groups based on the location of the tumour: retroperitoneal, cutaneous, osseous and vascular [15]. The vascular type is rare and often located in the inferior vena cava [16]. In the present case, the peculiar MSV involvement is suggestive of the leiomyosarcoma having a vascular origin and therefore could have been the primary cause of the thrombus. This hypothesis is supported by the ultrasonographic appearance of the vascular wall which appeared thickened and poorly differentiated from the surrounding tissues, and therefore was suspected to be the origin of the neoplasm. However, in absence of post-mortem examination this could not be confirmed.
In conclusion, this case report describes a unique presentation of leiomyosarcoma associated with compartment syndrome and MSV thrombophlebitis. The smooth muscle cells present in the tunica media of the MSV are considered the likely origin of the neoplasm. To the authors' knowledge, there are no previous cases showing these associations. This case highlights the importance of including neoplasia in the differential diagnoses of ECS and thrombophlebitis in horses.
Figures and Tables
 | Fig. 1Photographs of (A) the caudal and (B) craniomedial aspects of the left hindlimb of the mare on admission, demonstrating the marked swelling at the level of the crural region biaxially. Notice the pronounced appearance of the left medial saphenous vein (red arrows). |
 | Fig. 2Photographs of (A) the cranial, (B) lateral, (C) medial aspects of the left crural region swelling (arrows). Notice the distended superficial veins on the skin surface. |
 | Fig. 3(A) Transverse and (B) longitudinal Doppler ultrasonographic images of the left medial saphenous vein thrombophlebitis. Note the occluded vein with limited flow signal (arrowheads). The image (C) shows the thrombotic aneurism (arrows) at the level of the cranial vein wall. |
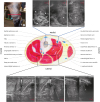 | Fig. 4Schematic illustration of the cross-sectional anatomy at the level of the highest distal third of the left crus corresponding to the red transverse line displayed in figure (A) and associated ultrasonographic images (blue and black rectangles). The ultrasound transducer was placed in a transverse orientation relative to the long axis of the limb. In the medial aspect, notice the thrombosed medial saphenous vein (arrows).V., vein; m., muscle; N., nerve
*The axial wall is not distinguishable from the surrounding tissue (arrowheads), which is of mixed echogenicity and ill-defined margins; †On the lateral aspect of the limb, note the similar ultrasonographic appearance of the soft tissue mass caudal to the lateral digital flexor muscle.
|
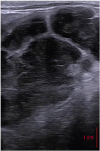 | Fig. 5Transrectal ultrasonographic image of the mixed echogenicity mass, located at the level of the most cranial aspect of the sacrum, suspected to be an enlarged sub-iliac lymph node. |
ACKNOWLEDGMENTS
The authors would like to thank the referring veterinary surgeon and acknowledge Marco Biffarella for the schematic illustration reported in Fig. 4.
References
1. Hurcombe SD, Slovis NM, Kohn CW, Oglesbee M. Poorly differentiated leiomyosarcoma of the urogenital tract in a horse. J Am Vet Med Assoc. 2008; 233:1908–1912.


2. Laugier C, Tapprest J, Foucher N, Doux N, George C, Longeart L, Le Net JL. Prevalence of equine tumours in 1771 horses examined post-mortem. Prat Vét Équine. 2004; 36:21–35.
3. MacGillivray KC, Graham TD, Parente EJ. Multicentric leiomyosarcoma in a young male horse. J Am Vet Med Assoc. 2003; 223:1017–1021.


4. Davis EG, Rush BR. Diagnostic challenges: equine thoracic neoplasia. Equine Vet Educ. 2013; 25:96–107.

5. Veraa S, Dijkman R, Klein WR, van den Belt AJ. Computed tomography in the diagnosis of malignant sinonasal tumours in three horses. Equine Vet Educ. 2009; 21:284–288.

6. Drew SJ, Meehan L, Reardon RJ, McGorum BC, Dixon PM, Del-Pozo J. Guttural pouch leiomyosarcoma causing nasopharyngeal compression in a pony. Equine Vet Educ. 2018; 30:64–69.

7. Grosås S, Østevik L, Revold T, Ottesen N, Ropstad EO. Uveal myxoid leiomyosarcoma in a horse. Clin Case Rep. 2017; 5:1811–1818.



8. Kawabata A, Del Piero F, Caserto BG, Langohr I. Metastatic leiomyosarcoma causing ataxia in a horse. J Equine Vet Sci. 2016; 43:23–27.

9. Maki LC, Kim SE, Winter MD, Kow KY, Conway JA, Lewis DD. Compartment syndrome associated with expansile antebrachial tumors in two dogs. J Am Vet Med Assoc. 2014; 244:346–351.


10. Bruniges N, Milner P, Bardell D. The use of multimodal analgesia in the management of suspected extremity compartment syndrome in the pelvic limb of a horse. Equine Vet Educ. 2019; 31:354–362.

11. Sullins KE, Heath RB, Turner AS, Stashak TS. Possible antebrachial flexor compartment syndrome as a cause of lameness in two horses. Equine Vet J. 1987; 19:147–150.


12. Norman WM, Williams R, Dodman NH, Kraus AE. Postanesthetic compartmental syndrome in a horse. J Am Vet Med Assoc. 1989; 195:502–504.

13. Nelson BB, Ragle CA, Barrett MF, Hendrickson DA. Use of a minimally invasive fasciotomy technique for treatment of antebrachial compartment syndrome in two horses. J Am Vet Med Assoc. 2015; 247:286–292.


14. Joo Han O, Kim JY, Kang M, Bae T, Lee T. Deep vein thrombosis associated with femur osteochondroma: report of a case. Ann Vasc Dis. 2009; 2:178–181.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download