INTRODUCTION
MATERIALS AND METHODS
Animal experiments
RNA preparation
cDNA microarray analysis
Pathway analysis
RESULTS
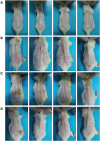 | Fig. 1The anti-atopic dermatitis effect of horse oil in DNCB-treated BALB/c mice. Skin lesions in the (A) control, (B) DNCB, (C) DNCB + AB, and (D) DNCB + horse oil treatment groups are shown.DNCB, 2, 4-dinitrochlorobenzene; AB, Atobarrier lotion.
|
 | Fig. 2Isolation of RNAs from the skin of mice. RNAs were isolated from the skin of control-, DNCB-, DNCB + AB-, and DNCB + horse oil-treated mice. (A) Migration pattern (electrophoretic trace), and (B) peak pattern (electropherogram) of RNAs are shown.DNCB, 2, 4-dinitrochlorobenzene; AB, Atobarrier lotion.
|
 | Fig. 3Scanned image of cDNA microarray chips. After labeling of cRNA, samples from the control-, DNCB-, DNCB + AB-, and DNCB + horse oil-treated mice were hybridized onto mouse oligo microarray slides, which were then scanned and analyzed.DNCB, 2, 4-dinitrochlorobenzene; AB, Atobarrier lotion.
|
Table 1
Differential expression of functional genes in mice skin treated with dinitrochlorobenzene only

Table 2
Differential expression of functional genes in mice skin treated with dinitrochlorobenzene and Atobarrier

Table 3
Differential expression of functional genes in mice skin treated with dinitrochlorobenzene and horse-oil

 | Fig. 4GO analysis of DNCB-treated mouse skin applied with/without AB or horse oil as a percentage of the total significant. The transcripts of the DNCB-treated mouse skin applied with/without AB or horse oil were classified into GO categories of (A) DNCB/control, (B) DNCB + AB/control, and (C) DNCB + horse oil/control based on their GO terms as a percentage of the total significant.GO, gene ontology; DNCB, 2, 4-dinitrochlorobenzene; AB, Atobarrier lotion.
|
Table 4
Up-regulated inflammatory genes in the skin of DNCB-treated mice and normalized by horse-oil

Table 5
Down-regulated inflammatory genes in the skin of DNCB-treated mice and normalized by horse-oil

 | Fig. 5PPI network constructed for DEGs identified in mouse skin treated with DNCB and horse oil. Representative image of the PPI network is shown. Bold lines represent the strongly connected genes associated with inflammation. Different colors indicate DEGs; red indicates up-regulated gene expression and blue indicates down-regulated gene expression.PPI, protein-protein interaction; DEG, differentially expressed gene; DNCB, 2, 4-dinitrochlorobenzene.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download