1. Ziffer AM, Scopp IW, Beck J, Baum J, Berger AR. Profound bleeding after dental extractions during dicumarol therapy. N Engl J Med. 1957; 256:351–353. PMID:
13419000.

2. Cannon PD, Dharmar VT. Minor oral surgical procedures in patients on oral anticoagulants--a controlled study. Aust Dent J. 2003; 48:115–118. PMID:
14649401.
3. Bouloux GF, Perciaccante VJ. Massive hemorrhage during oral and maxillofacial surgery: ligation of the external carotid artery or embolization? J Oral Maxillofac Surg. 2009; 67:1547–1551. PMID:
19531434.

4. Bajkin BV, Popovic SL, Selakovic SD. Randomized, prospective trial comparing bridging therapy using low-molecular-weight heparin with maintenance of oral anticoagulation during extraction of teeth. J Oral Maxillofac Surg. 2009; 67:990–995. PMID:
19375008.

5. Costa FW, Rodrigues RR, Sousa LH, Carvalho FS, Chaves FN, Fernandes CP, et al. Local hemostatic measures in anticoagulated patients undergoing oral surgery: a systematized literature review. Acta Cir Bras. 2013; 28:78–83. PMID:
23338118.

6. Scully C, Wolff A. Oral surgery in patients on anticoagulant therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:57–64. PMID:
12193895.

7. Medina JB, Andrade NS, de Paula, Bezinelli L, Franco JB, Gallottini M, et al. Bleeding during and after dental extractions in patients with liver cirrhosis. Int J Oral Maxillofac Surg. 2018; 47:1543–1549. PMID:
29705406.

8. Rasaratnam L, Chowdary P, Pollard D, Subel B, Harrington C, Darbar UR. Risk-based management of dental procedures in patients with inherited bleeding disorders: Development of a Dental Bleeding Risk Assessment and Treatment Tool (DeBRATT). Haemophilia. 2017; 23:247–254. PMID:
28092925.

9. Sprenker C, Omar HR, Powless RA, Mangar D, Camporesi E. Massive oral bleeding after full-mouth extraction in a patient with B-cell lymphocytic leukemia/small lymphocytic lymphoma reversed with recombinant activated factor VII. J Am Dent Assoc. 2016; 147:142–145. PMID:
26562728.

10. Krishnan B, Shenoy NA, Alexander M. Exodontia and antiplatelet therapy. J Oral Maxillofac Surg. 2008; 66:2063–2066. PMID:
18848103.

11. Kämmerer PW, Frerich B, Liese J, Schiegnitz E, Al-Nawas B. Oral surgery during therapy with anticoagulants-a systematic review. Clin Oral Investig. 2015; 19:171–180.

12. Madrid C, Sanz M. What influence do anticoagulants have on oral implant therapy? A systematic review. Clin Oral Implants Res. 2009; 20 Suppl 4:96–106. PMID:
19663955.

13. Wahl MJ, Pinto A, Kilham J, Lalla RV. Dental surgery in anticoagulated patients--stop the interruption. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015; 119:136–157. PMID:
25577414.

14. Eichhorn W, Burkert J, Vorwig O, Blessmann M, Cachovan G, Zeuch J, et al. Bleeding incidence after oral surgery with continued oral anticoagulation. Clin Oral Investig. 2012; 16:1371–1376.

15. Morimoto Y, Nakatani T, Yokoe C, Kudo C, Hanamoto H, Niwa H. Haemostatic management for oral surgery in patients supported with left ventricular assist device--a preliminary retrospective study. Br J Oral Maxillofac Surg. 2015; 53:991–995. PMID:
26362416.
16. Vezeau PJ. Topical hemostatic agents: what the oral and maxillofacial surgeon needs to know. Oral Maxillofac Surg Clin North Am. 2016; 28:523–532. PMID:
27745620.
17. Rossmann JA, Rees TD. A comparative evaluation of hemostatic agents in the management of soft tissue graft donor site bleeding. J Periodontol. 1999; 70:1369–1375. PMID:
10588501.

18. Ozcan M, Ucak O, Alkaya B, Keceli S, Seydaoglu G, Haytac MC. Effects of platelet-rich fibrin on palatal wound healing after free gingival graft harvesting: a comparative randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2017; 37:e270–e278. PMID:
28817141.

19. Arora RR, Rai F. Antiplatelet intervention in acute coronary syndrome. Am J Ther. 2009; 16:e29–e40. PMID:
19092648.

20. Su Q, Li C, Long F, Chen B, Wan Z, Wu Y, et al. Effects of a health promotion program on medication adherence to antiplatelet therapy among ischemic stroke patients in Hainan Province, China. Vascular. 2017; 25:242–248. PMID:
27580820.

21. Pototski M, Amenábar JM. Dental management of patients receiving anticoagulation or antiplatelet treatment. J Oral Sci. 2007; 49:253–258. PMID:
18195506.

22. Nathwani S, Martin K. Exodontia in dual antiplatelet therapy: the evidence. Br Dent J. 2016; 220:235–238. PMID:
26964594.

23. Li L, Zhang W, Yang Y, Zhao L, Zhou X, Zhang J. Dental management of patient with dual antiplatelet therapy: a meta-analysis. Clin Oral Investig. 2019; 23:1615–1623.

24. Napeñas JJ, Oost FC, DeGroot A, Loven B, Hong CH, Brennan MT, et al. Review of postoperative bleeding risk in dental patients on antiplatelet therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 115:491–499. PMID:
23332510.

25. Bacci C, Berengo M, Favero L, Zanon E. Safety of dental implant surgery in patients undergoing anticoagulation therapy: a prospective case-control study. Clin Oral Implants Res. 2011; 22:151–156. PMID:
20946205.

26. Scully C, Hobkirk J, Dios PD. Dental endosseous implants in the medically compromised patient. J Oral Rehabil. 2007; 34:590–599. PMID:
17650169.

27. Febbo A, Cheng A, Stein B, Goss A, Sambrook P. Postoperative bleeding following dental extractions in patients anticoagulated with warfarin. J Oral Maxillofac Surg. 2016; 74:1518–1523. PMID:
27186873.

28. Gualandro DM, Yu PC, Calderaro D, Marques AC, Pinho C, Caramelli B, et al. II Guidelines for perioperative evaluation of the Brazilian Society of Cardiology. Arq Bras Cardiol. 2011; 96(3 Suppl 1):1–68.
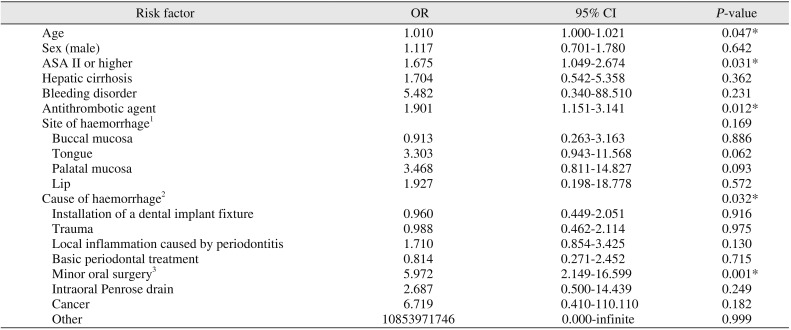
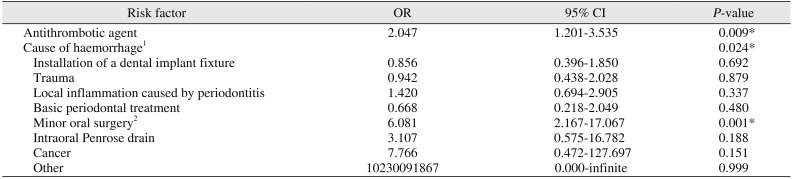
29. Morimoto Y, Niwa H, Minematsu K. Risk factors affecting postoperative hemorrhage after tooth extraction in patients receiving oral antithrombotic therapy. J Oral Maxillofac Surg. 2011; 69:1550–1556. PMID:
21277059.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download