INTRODUCTION
Pelvic sonography via transvaginal or transrectal approach is used as a first-line investigation in patients with abnormal uterine bleeding and it can provide not only anatomical assessment but also physiological changes accompanied with menstrual cycle [
1]. Various gynecologic conditions can make endometrium thick above the normal sonographic reference range considering the patient's menstrual cycle and menopausal status. There is no widely accepted threshold for abnormal endometrial thickness in premenopausal women, although typically diffuse homogeneous thickening greater than 16 mm in the secretory phase is used [
2]. For postmenopausal women, the endometrial thickness thresholds are known to be 4 mm or 5 mm [
34]. Even asymptomatic postmenopausal women, an endometrial thickness more than 10 mm carries a 5.8% risk of endometrial cancer [
5]. In addition to the finding of thick endometrium, multiple foci of cystic change in endometrium accompanied with it and it is called as a honeycomb or “Swiss cheese” appearance. The most commonly known endometrial conditions which can cause thick endometrium with “Swiss cheese” appearance in sonography are endometrial polyp, hyperplasia, and endometrial cancer [
6]. Hydatidiform mole and arterio-venous malformation (AVM) also were reported to be related with thick endometrial sonographic finding [
7]. However, various gynecologic conditions such as submucosal leiomyoma with degeneration, adenomyomatous endometrial polyp, and pseudocystic endometrial change associated with tamoxifen use, progesterone associated endometrial change, pyometra, retained placenta, and uterine synechiae also can be manifested as similar finding of thick endometrium with “Swiss cheese” appearance in transvaginal sonography [
89]. Therefore, we want to present several cases of diverse endometrial pathology with similar sonographic finding of thick endometrium with “Swiss cheese” appearance in pre- and postmenopausal women to analysis of various gynecologic situations with the similar transvaginal sonographic findings of thick uterine endometrium with “Swiss cheese” appearance.
Go to :

RESULTS
Diverse gynecologic conditions, such as submucosal leiomyoma with degeneration, adenomyomatous endometrial polyp, pseudocystic endometrial change associated with tamoxifen use, progesterone associated endometrial change (PAEC), pyometra, retained placenta, and uterine synechiae showed as similar findings of thick uterine endometrium with “Swiss cheese” appearance in transvaginal sonography. The clinical and transvaginal sonographic findings with or without hysteroscopic findings of these cases were summarized in
Table 1 and the sonographic and hysteroscopic images of the patients are in
Figure 1. In postmenopausal women group, 7 cases of malignant cases, 6 cases of endometrial cancer, and 1 case of cervical cancer were the leading causes and 3 cases of endometrial polyp and 1 case of degenerated submucosal myoma were followed. Two cases of endometritis and the others were atrophy without evidence of endometrial pathology. In premenopausal women group, there was no case of malignant lesion and 1 case of simple hyperplasia and 1 case of complex hyperplasia were noted. One case of acute pyometritis, 1 case of chronic endometritis, and 8 cases of normal endometrial tissue were noted. Nine out of 12 cases in premenopausal women and 19 out of 57 cases in postmenopausal women did not show vaginal bleeding. The other cases had vaginal bleeding. All 2 cases of endometrial hyperplasia in premenopausal women showed vaginal bleeding and 6 out of 7 malignant cases in postmenopausal women accompanied vaginal bleeding.
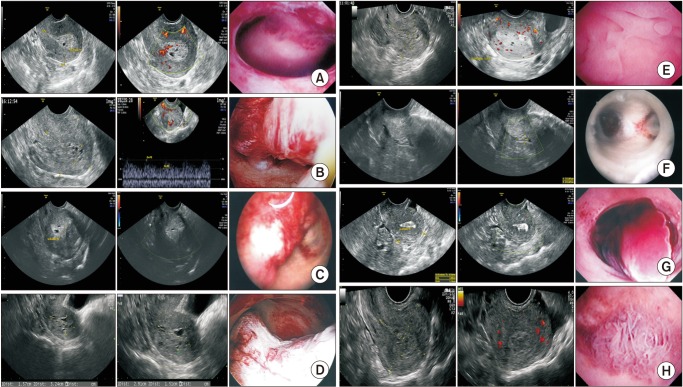 | Fig. 1Gray-scale image, color Doppler sonographic image, and hysteroscopic image (from left to right) of patients with thick endometrium with “Swiss cheese” appearance on transvaginal sonography.
|
Table 1
Summary of clinical and sonographic findings of cases and final diagnosis pathologically confirmed
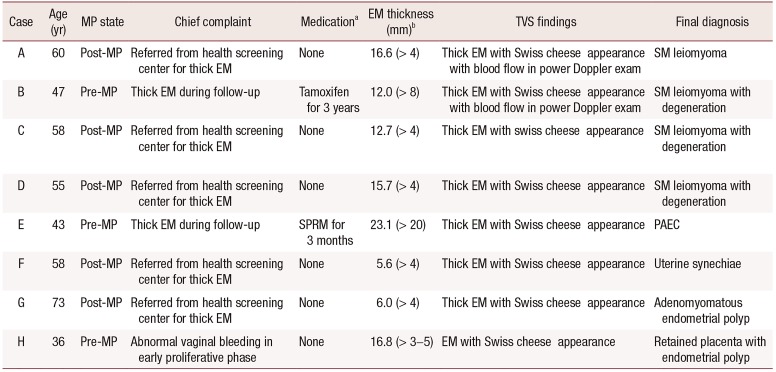
|
Case |
Age (yr) |
MP state |
Chief complaint |
Medicationa
|
EM thickness (mm)b
|
TVS findings |
Final diagnosis |
|
A |
60 |
Post-MP |
Referred from health screening center for thick EM |
None |
16.6 (> 4) |
Thick EM with Swiss cheese appearance with blood flow in power Doppler exam |
SM leiomyoma |
|
B |
47 |
Pre-MP |
Thick EM during follow-up |
Tamoxifen for 3 years |
12.0 (> 8) |
Thick EM with Swiss cheese appearance with blood flow in power Doppler exam |
SM leiomyoma with degeneration |
|
C |
58 |
Post-MP |
Referred from health screening center for thick EM |
None |
12.7 (> 4) |
Thick EM with swiss cheese appearance |
SM leiomyoma with degeneration |
|
D |
55 |
Post-MP |
Referred from health screening center for thick EM |
None |
15.7 (> 4) |
Thick EM with Swiss cheese appearance |
SM leiomyoma with degeneration |
|
E |
43 |
Pre-MP |
Thick EM during follow-up |
SPRM for 3 months |
23.1 (> 20) |
Thick EM with Swiss cheese appearance |
PAEC |
|
F |
58 |
Post-MP |
Referred from health screening center for thick EM |
None |
5.6 (> 4) |
Thick EM with Swiss cheese appearance |
Uterine synechiae |
|
G |
73 |
Post-MP |
Referred from health screening center for thick EM |
None |
6.0 (> 4) |
Thick EM with Swiss cheese appearance |
Adenomyomatous endometrial polyp |
|
H |
36 |
Pre-MP |
Abnormal vaginal bleeding in early proliferative phase |
None |
16.8 (> 3–5) |
EM with Swiss cheese appearance |
Retained placenta with endometrial polyp |

Go to :

DISCUSSION
The most important endometrial conditions which should be differentiated are endometrial hyperplasia and endometrial cancer. These two conditions can be manifested as thick endometrium with “Swiss cheese” appearance in pelvic sonography [
6]. The clinical characteristics including abnormal uterine bleeding, postmenopausal state, unopposed estrogen, estrogen secreting ovarian tumor, and obesity are the well-known risk factor of these conditions. More than 90% of endometrial cancer occurs after age 50, with the most common symptom by far being abnormal vaginal bleeding [
10]. For differential diagnosis of these pre-malignant and malignant endometrial lesions with other benign conditions before making decision on endometrial tissue biopsy, we sometimes perform additional diagnostic imaging. In case of suspected endometrial pathologic condition, the saline infusion sonography (SIS) is considered as an option for more clear visualization of endometrial condition. Hysteroscopy, especially office flexible hysteroscopy, has become the new good option for this purpose because of its ability to visualize directly the endometrium and perform directed biopsies as indicated. As office-based hysteroscopy becomes more practical and widespread, the technique may become more cost-effective [
11]. In addition to that, office hysteroscopy usually causes no or minimal discomfort to patients and shorter time compared with SIS. An evaluation plan using transvaginal sonography as the initial screening evaluation followed by endometrial biopsy or, more likely, hysteroscopy is likely to become the standard of care [
11]. Therefore in our gynecologic clinic, in case of suspected endometrial disease other than endometrial hyperplasia or endometrial cancer, transvaginal sonography as the initial screening evaluation followed by office flexible hysteroscopy in the same day is become increasing.
In terms of thick uterine endometrium with “Swiss cheese” appearance in transvaginal sonography, there were few reports describing the underlying endometrial pathologies other than commonly well-known conditions such as endometrial hyperplasia, endometrial cancer, hydatidiform mole, and AVM [
12]. Cohen et al. [
11] reported a series of cases with submucosal fibroids undergoing cystic degeneration showed a distinct sonographic “Swiss cheese” pattern and erroneously diagnosed as endometrial hyperplasia.
In our case series, even in postmenopausal women who are not taking postmenopausal hormone therapy with very thick endometrium measured to be over 12 mm, the possibility of endometrial hyperplasia or cancer may be low unless presented with abnormal vaginal discharge, vaginal spotting, or bleeding. Therefore, office hysteroscopy with or without pipelle biopsy may be the better options for the next step evaluation after transvaginal sonography instead of dilatation and curettage (D&C) requiring anesthesia and abstinence of food and drink for several hours because the pipelle biopsy is known to be comparable to the D&C method in evaluating endometrial pathology [
131415]. Endometrial biopsy with D&C method may be reserved in case of thick endometrium with clinically suspected in an elderly postmenopausal woman with vaginal discharge or bleeding.
We have to differentiate the thickening endometrium itself and the presence of endometrial mass when we encounter thick endometrial finding on ultrasonography. The thorough examination of vaginal sonography can reveal hypoechoic space between normal appearing hyperechoic endometrium and “Swiss cheese” like endometrial mass (
Fig. 2). That finding suggests the thick endometrium is caused by the presence of endometrial mass and not a real thickening of endometrium such as endometrial hyperplasia, pyometra, tamoxifen user, or PAEC. SIS is also helpful to differentiate the thick endometrium and endometrial mass.
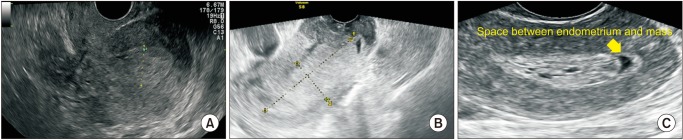 | Fig. 2The thorough examination of vaginal sonographic image can reveal space between normal appearing endometrium and “Swiss cheese” like endometrial mass. (A) Endometrial hyperplasia. (B) Endometrial cancer. (C) Submucosal leiomyoma with cystic degeneration.
|
Thorough reviewing the medications which the patients are taking is important, the reason for this is medications especially tamoxifen and selective progesterone receptor modulator (SPRM) can make a change in endometrium to be multicystic endometrial thicening. In terms of PAEC, some patients who are taking SPRM such as ulipristal acetate (UPA; Inisia®) for medical treatment for uterine myoma with menorrhagia can experience endometrial change which is not a pathologic finding and returned to normal endometrium. The endometrial changes proven microscopically contained abortive subnuclear vacuolization, occasional mitoses, and apoptosis [
16]. Small and large cysts and focal septum like thickening are observed in hysteroscopy in our experience and it was in accordance with previously reporting finding [
17]. This PAEC can also be observed in magnetic resonance imaging (MRI) and fat suppression mode was more clearly showed this change than in T1-weighted or T2-weighted image and disappeared after spontaneous menstruation finishing 1 cycle of UPA treatment (
Fig. 3).
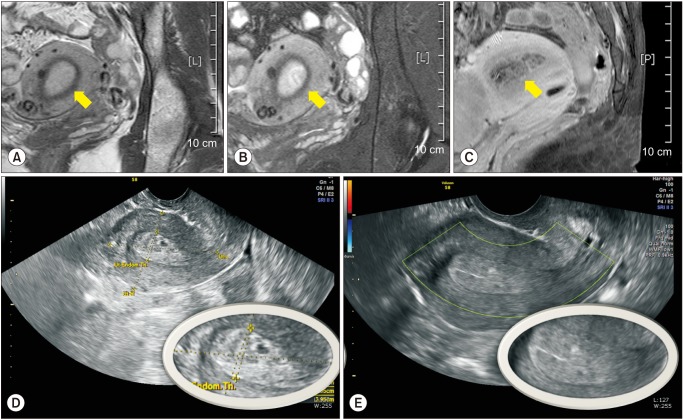 | Fig. 3The magnetic resonance imaging (MRI) of progesterone associated endometrial change (PAEC). (A) T1-weighted image of MRI. (B) T2-weighted image of MRI. (C) Fat suppression image of MRI. The transvaginal sonographic image of PAEC before (D) and after (E) menstruation showed the “Swiss cheese” appearance of (D) is disappeared in (E) showing triple laminar feature of normal endometrium.
|
In case of tamoxifen user, the normal range of endometrium was reported as below 8 mm and most cases of endometrial changes related with tamoxifen were reported to a cystic endometrial changes or endometrial polyp [
16]. Rarely the risk of endometrial proliferation, hyperplasia, or cancer, and uterine sarcoma are associated with tamoxifen. Although the risk of endometrial cancer is comparable to the control patients who are taking placebo, women with abnormal vaginal bleeding, bloody vaginal discharge, staining, or spotting who are taking tamoxifen should be investigated [
17]. If a blind endometrial biopsy was negative in case of multicystic endometrial thickening, office hysteroscopic examination will be the good next step for avoiding repeat D&C under anesthesia. This is especially helpful in patients without symptoms such as vaginal bleeding or discharge. It would be better to perform D&C under the setting of operative hysteroscopy for resection of endometrial polyp or degenerated submuscosal myoma, which usually cannot be clearly resected with D&C (
Fig. 4).
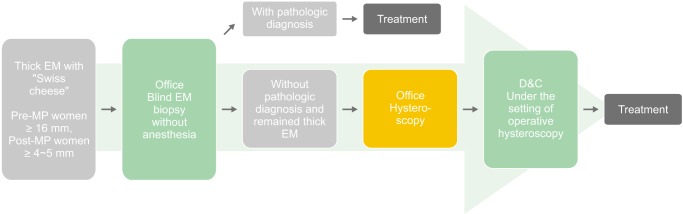 | Fig. 4Proposed diagnostic algorithm for managing thick uterine endometrium with “Swiss cheese” appearance. EM: endometrium, MP: menopause, D&C: dilatation and curettage.
|
In conclusion, we have to take into consideration these diverse conditions when we encounter the sonographic finding of thick endometrium with “Swiss cheese” appearance. We can make a tentative diagnosis only after thorough reviewing the medical and surgical history and chief complaint of the patient for cases with similar sonographic finding of uterine endometrium.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download