This article has been
cited by other articles in ScienceCentral.
Abstract
Absence of left kidney was noted during routine anatomy dissection of a male cadaver of South Indian origin. On examination of the abdomen and pelvic cavities; an ovoid mass of tissue was found in the pelvis, anterolateral to the sacrum. Further dissection revealed the presence of an ectopic left side kidney. The ectopic kidney was lying inferior to the sigmoid colon and anterior to the bifurcation of left common iliac vessel. It was supplied by numerous aberrant vessels from the terminal part of abdominal aorta. One of the renal veins which drain the ectopic kidney was found to be persisting subcardinal vein and it is a novel finding. Such ectopic pelvic kidneys are susceptible to blunt trauma, iatrogenic injuries as well as pathologic manifestations.
Keywords: Ectopic pelvic kidney, Aberrant renal arteries, Subcardinal vein, Suprarenal gland
Introduction
In humans, metanephric kidney development begins at day 28 after fertilization, when the ureteric bud sprouts from the distal part of the mesonephric duct or Wolffian duct [
123]. The fetal metanephros-mesenchymal cells that originate from the intermediate mesoderm [
3] is located at vertebral level S1–S2, whereas the definitive adult kidney is located at vertebral level T12–L3. Migration of kidneys to the adult position (L1) is due to disproportionate growth of the embryo caudal to the metanephros. Failure of migration results in renal ectopia [
4]. The incidence of renal ectopia is between 1 in 2,200 birth to 1 in 3,000 and found more in males [
5]. Rate of left side renal ectopia is found more than the right. We report a unique case of left pelvic kidney combined with persisting subcardinal vein and in our knowledge, this is the first report regarding such association.
Case Report
Absence of left kidney was noticed during routine anatomy dissection of a male cadaver of South Indian origin. On further examination of the abdomen and pelvic cavities; an ovoid mass of tissue was found in the pelvis, covered with peritoneum, at the pelvic brim, anteromedial to the psoasmajor muscle.
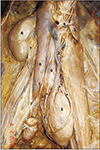
The sigmoid colon was displaced upwards and fixed above the ovoid mass. The second part of the sigmoid colon; lying vertical as well as towards the right of this mass was crossed anteriorly by the terminal part of the root of the mesentery of small intestine. Small intestine along with its mesentery was removed in further dissection. The peritoneum covering the anterior surface of the ovoid mass was removed and presence of the ectopic pelvic kidney was confirmed.
The ectopic kidney was lying inferior to the displaced sigmoid colon and anterior to the bifurcation of left common iliac vessel. The sigmoid colon was long and broad. Its first part was above the ectopic kidney and it was crossing the renal vessels (
Fig. 1).
The ectopic kidney was measured 8×4.5 cm, with an anteromedial and posterolateral surface. The posterolateral surface was related to the common iliac vessels and its branches/tributaries. and this surface was grooved by those vessels. Anteromedial surface does not show a renal sinus. The renal vessels and renal pelvis were seen on this surface. There were three main arteries to nourish the ectopic kidney. The first two arteries were arising from the left lateral aspect of terminal part of aorta and the third artery was arising from the anterior aspect of the bifurcation of aorta. Among the first two arteries, the proximal one was short and it entered into the kidney from its upper pole. Before it enters the kidney, it gave a branch and that branch entered into kidney through the upper part of its anterolateral border. The second of the first two arteries (emerging from terminal part of aorta) was very long and it was running along the anterolateral border of the upper part of the kidney and in the middle of the kidney, it crossed from lateral to medial to reach anteromedial surface/hilum. On the way, it gave a branch which enters directly into the kidney near its anterolateral border. The third renal artery was arising from the bifurcation of aorta and before it reaches the kidney it divided into two—the right one entered directly into the kidney while the left one divided into two small branches and entered the kidney from its anteromedial aspect.
There were two major renal veins to drain the kidney; emerging from its anteromedial surface. Among the two veins—the short vein was running along the third artery towards the right and drain into inferior vena cava (IVC) at its formation i.e., at the union of left and right common iliac vessels. The long vein was formed by two small veins from the ectopic kidney and after the formation, it was running along the lateral side of abdominal aorta and medial to psoas major muscle. While it was running upwards, it received two tributaries—left gonadal vein and another vein from lateral pelvic wall, emerged in between psoas major muscle and common iliac vessels. This renal vein joined to the IVC opposite to the right renal vein. Before it joined to IVC, it received two tributaries one from the left suprarenal gland and another vein emerged from the medial side of psoas major; lateral to aorta.
The pelvis of the ureter was seen in the inferior part of the anteromedial surface. It was formed by the union of two major calyces which were also visible in the anteromedial surface of the ectopic kidney. Pelvis continued as ureter which leaves the kidney and reaches the lateral pelvic wall. There it enters into the bladder through its lateral angle.
Left suprarenal gland was present in the lumbar region, at the level of origin of superior mesenteric artery. It was oval and flat in shape with two surfaces—lateral and medial, two borders—posterolateral and anteromedial. Medial surface was related to left crus of diaphragm superomedially, psoas major—inferiorly and diaphragm itself laterally. Inferior part of lateral surface was related to the body of pancreas and the upper part was covered with peritoneum. A suprarenal vein was emerged from the medial part of this surface and joined with inferior phrenic vein and then terminated into left renal vein. Suprarenal arteries where two in number—superior and inferior. Superior suprarenal artery has a common origin with inferior phrenic artery. Inferior phrenic artery was taking origin from the left lateral side of abdominal aorta. Both suprarenal arteries were reached the suprarenal gland from its medial surface.
Discussion
Ectopic kidneys are formed when the mature kidney fails to reach its normal position and found in locations such as the pelvic, iliac, abdominal, and thoracic regions [
2]. Such abnormally placed kidneys are prone for blunt trauma, hydronephrosis and renal tumors [
5]. In a meta-analysis of 120 patients with all blunt renal trauma cases, ectopic kidneys constituted 7% of cases and in most of the ectopic kidney cases had other nephrological conditions such as renal cysts, hydronephrosis, and renal tumors [
6].
In the present case, it was found in the pelvis anterior to the bifurcation of left iliac vessel. Since it failed to migrate, the hilum of the ectopic kidney was facing anteromedially. There was no renal sinus, renal vessels and pelvis of ureter along with its calyces were found emerging from anteromedial surface. Presence of such extrarenal calyces and renal pelvis are prone for ureteropelvic junction obstruction (UPJO) and hydronephrosis [
78]. It was said that such ectopic renal tissue will be nourished by numerous anomalous vessels [
9]. In the present case also, it was supplied by three such anomalous arteries originated from the terminal part of abdominal aorta.
In the present case, the ectopic kidney was drained by two veins one was accompanying one of the renal artery towards the right and terminated in the IVC at its formation. While the second renal vein was long and ascend to the lumbar region to drain into the IVC opposite to the right renal vein. The course and appearance of this renal vein are similar to the embryonic subcardinal vein. In fetal life initially the body wall was drained by posterior cardinal veins and the developing mesonephros drain into it. Later subcardinal veins appear ventromedial to mesonephros and get connected with the posterior cardinal veins through numerous anastomoses. Eventually both postcardinal and the subcardinal veins drain the mesonephric kidneys through numerous small side branches. When the metanephros appears in fifth week of intrauterine life, the subcardinal veins assumes the venous drainage of it. At the cranial end, the subacardinal veins receive suprarenal veins. The caudal part of the subcardinal veins gets incorporated into the gonadal veins [
10]. The above said embryological features are found in the present case and suggest that it is the persisting subcardinal vein which exists during early embryonic life. In the present case, the left gonadal vein, a vein from the dorsal body wall of lumbar region and the left suprarenal vein also found to drain into this long renal vein and these features also suggest that it is the persisting subcardinal vein.
The present case of ectopic pelvic kidney is unique. The vein which drains this ectopic kidney was found to be persisting subcardinal vein and such finding was not reported earlier. Ectopic pelvic kidneys are susceptible to blunt trauma, iatrogenic injuries as well as pathologic manifestations. Knowledge of ectopic position of kidney should be bear in mind while performing surgical removal of abnormal masses from abdomen or pelvis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download