Abstract
Menstrual cycle is controlled by luteinizing hormone (LH) and follicle-stimulating hormone of anterior pituitary and regulated by gonadotropin-releasing hormone of hypothalamus. Any disturbance to this regulatory mechanism alters the pulsatile release of these hormones, especially LH; leads to polycystic ovarian syndrome (PCOS). Changes in vaginal cytology are used to interpret the changes in hormonal levels and modifications in estrous cycle. The aim of this study is to compare the pattern of vaginal cytology and body mass among PCOS rats which are treated with metformin and Cynodon dactylon. Twenty-four Wistar rats were selected and divided into four groups: control, induced, treatment, and referral. PCOS was induced in all groups except controls by giving letrozole through oral gavage for 21 days. After inducing PCOS, the referral and treatment group were treated for PCOS with metformin and C. dactylon respectively for next 21 days. Vaginal smear of all the groups were taken every day from day one and screened for estrous cycle. The body mass of the animals was measured on days 1, 21, and 42. Animals were sacrificed after 24 hours of the last dose and the reproductive organs were dissected out and weighed. Results of the study show the estrous cycle begins to revert after 1-week administration of C. dactylon; while the changes were slower in referral group. There was a rapid decrease in the body mass as well as reproductive organs among the treatment and referral group compared to that of induced and control. Finding of this study suggests that C. dactylon treats PCOS better than metformin.
The menstrual cycle is a standard reproductive cycle that takes place in a female lifetime from the era of puberty to menopause. Two significant hormones control this menstrual cycle, namely follicular stimulating hormone and luteinizing hormone (LH), which are regulated by the release hormone of gonadotropin. Gonadotropin-releasing hormone of hypothalamus stimulates the follicle-stimulating hormone and LH in a pulsative frequency. It causes polycystic ovarian syndrome (PCOS) when this pulse frequency is disrupted [123].
PCOS is identified by Rotterdam criteria using the following signs and symptoms: (1) hyperandrogenism and/or hyperandrogenemia, (2) oligo-ovulation, (3) exclusion of known disorders, such as Cushing's syndrome, hyperprolactinemia, congenital adrenal hyperplasia and (4) polycystic ovaries on ultrasound [456].
Studies have been performed to treat PCOS using animal models for reasons such as shorter life span and differences in the estrous cycle, endocrine changes, and morphological similarities. PCOS is induced by the use of oral drug letrozole for 21 to 28 days or intramuscular injection estradiol valerate. In order to verify PCOS in rodents, estrous cycle is noted from day 1 to 21, as well as changes in ovulation phases determined by amount of cornified cells, nucleated epithelial cell leukocytes in vaginal smear morphology. In the estrous cycle, the animals remain static in the diestrus phase, which is predominantly leukocyte cells [78]. Numerous studies validate the diestrus phase in PCOS, but none have disclosed the estrous cycle processing pattern in rodents. The above research was done to fill this lacuna. Evaluation of vaginal cytology is particularly used to determine the mating period of rodonts and to reduce pseudopregnancy [9]. A regular estrous cycle shows consistent internal change in vaginal epithelial cytology. This is directly related to the phases of vaginal, uterine and ovarian changes in reproductive hormones and their impact on the target organs [10]. Thus, vaginal cytology can reveal the alteration in the steroid genic condition of the rodent models and this can be used as a resource for detecting PCOS in animal models to protect the mortality of the species.
Cynodon dactylon or Bermuda grass is seen in moderate climate all over the world between south and north latitudes. C. dactylon is a stoloniferous, hardy perennial grass, very much variable with long rapid growing, rooting at nodes, forming a dense tuft on the top of the soil [11]. C. dactylon is widely used for traditional medical practice in India [12]. Crude extract of this plant is used for treatment of cancer [13], obesity, diabetic [14] gastric ulcers [15], etc. There is also evidence for its antihyperlipedemic [12], hepatoprotective [16] antimicrobial [1718], and anti-atherosclerotic [19] properties of this plant.
The study was designed in Sri Lakshmi Narayana Institute of Medical Sciences, Pondicherry and carried out in JKK Munirajah Medical Research Foundations College of Pharmacy, Tamil Nadu, after obtaining due institutional, animal ethical clearances. Twenty-four Wistars albino rats were taken and divided into four groups of six animals in each. The groups were follows: control group, induced (PCOS) group, referral group (metformin 100 mg/kg), and treatment group (C. dactylon 500 mg/kg).
C. dactylon plant was collected from the campus of Sri Lakshmi Narayana Institute of Medical Sciences, Puducherry. One hundred grams of plant powder was mixed with 1,000 ml of distilled water and heated until boiling. The mixture was filtered and lyophilization was done.
Each animal was taken off the cage, a wet cotton swab was inserted into the vagina of the animal while carefully holding the tail in one hand. The wet cotton swab was gently rotated and removed out of the animal. Using the wet cotton swab, a smear was created on a clean grease-free microscope slide. The slides were air-dried and stained with methylene blue or crystal violet stain and observed under a binocular microscope to identify different stages of estrous cycle [820].
The proestrous is defined by the existence between cells of small, round, nucleated epithelial cells with resemblance in form. They are also numerous in numbers. The nuclei are basophilic, and the cells are seen in clusters (Fig. 1). There are also mostly nucleated and some cornified epithelial cells.
The estrous is defined by extensive cornified epithelial cells without nuclei and some well-developed nucleated epithelial cells (Fig. 2).
The metaestrous is defined by the presence of both predominately cornified epithelium with nucleus and without nucleus as well as a few neutrophils (Fig. 3). Mostly cornified epithelial cells, neutrophils, and some nucleated epithelial cells are present.
Diestrous is featured by having a greater number of neutrophils and lesser number of cornified epithelial cells (Fig. 4).
The selected animals weighed between 125 and 150 g and were in an estrous cycle. All of the animals had free access to food and water. Twenty-four rats were examined in the every-day vaginal cycle. Animals in groups 2 to 4 were administered letrozole with oral feeding needle for 21 days in the first stage (induced). Vaginal smear was examined to confirm development of PCOS. In the second stage (treatment), 22–42 days, the animals in groups 3 and 4 were treated with Bermuda grass extract and metformin respectively. The animals were weighed periodically on first day of induction, on the 21st day and on the 42nd day. After 24 hours from the last dose of Bermuda grass extract and metformin the animals were anesthetized, decapitated and dissected. The ovaries and uteruses were meticulously removed and weighed using three digital accurate weighting balances. The mean value was calculated, and graph plotted using Excel document.
Throughout the experiment, the cyclic changes were regular in the control group indicating proestrous, estrous, metaestrous, and diestrous phases at regular time periods. Fig. 5 shows the alterations taking place in this group's estrous cycle.
Except for the first two cycles, the animals stayed in diestrous phase. However, from the 9th day onwards, the estrous cycle was delayed, and the animals remained in diestrous stage. Moreover, on 31st and 32nd day the animals progressed to proestrous stage and on the 33rd day it changed to diestrous stage. Fig. 6 shows the modification taking place in the estrous cycle of this group.
The estrous cycle was regular in initial stage, and on 10th day the animals were in diestrous phase of the cycle. On 22nd day the animal was induced to develop PCOS. On 25th day, the cycle changed to proestrous and remained so for 2 days, changed to diestrous and continued for two days and then the estrous cycle was regular from 29 to 42nd day. Fig. 7 shows the changes taking place in the estrous cycle of this group.
The estrous cycle was normal till 13th day and continued to be stable in diestrous phase till 22nd day. On the 26th day, proestrous phase was observed and continued for 3 days and transformed to estrus for 1day and thereafter continued with diestrous. The estrous cycle stayed regular from 36th day to 42nd day. Fig. 8 shows the changes taking place in the estrous cycle of this group.
On observing the estrous cycles of the said four groups, the drug group was found to have early changes in the estrous cycle compared to that of metformin group.
In the control group, weight increased gradually throughout the period of experiment (from day 1 to 42). On the other hand, in the induced group weight increased rapidly up to day 21 and then gradually increased. Contrarily, in the metformin group and drug group, weight increased rapidly till day 21 and then declined till day 42 (Fig. 9).
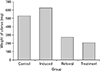
The weights of right and left ovaries were found to be less in control group when compared to induced group. Likewise, weights of ovaries in referral group and treatment group were less than that of the induced group. The ovarian weight in the treatment group is also less than the induced group. Overall, weight of the ovaries in the induced group is the highest compared to the other three groups (Fig. 10).
Normally the estrous cycle comprises of four phases: proestrous, estrous, metaestrous, and diestrous. A full cycle of the estrous takes 4 to 5 days [8]. Letrozole is an inhibitor of aromatase which induces PCOS in animal models. In most studies, letrozole is used to induce PCOS in rodent models and corresponding changes in the cytological features of vaginal smear, indicating the estrous cycle to be arrested in diestrous phase [2122]. In the existing research, vaginal cytology was used as an indicator of inversion of the estrous cycle in contrast between drug group and metformin group. Comparing the mean body weight, there was an increase in all groups except the control group on the 21st day. However, after day 21, there was a fast reduction in body weight in treatment and referral group. The weight of the right and left ovaries and the weight of the uterus was found to be increased in the induced group and decreased in treatment and referral groups. These changes have also been observed in other studies [2324252627]. Nevertheless, the uterine mass was reduced in the treatment group compared to that of the referral group in our study. This is mainly due to the antihyperlipedemic [12] and decrease in insulin resistance [14] activity of the C. dactylon.
The restoration of esterous cycles occurred a day earlier in PCOS animal models treated with water-soluble C. dactylon (Bermuda grass) extract compared to that of metformin-treated group.
Figures and Tables
 | Fig. 1Proestrous stage with nucleated epithelium (circle) and cornified epithelium (arrow) (A, ×40; B, ×10). |
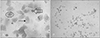 | Fig. 3Metaestrous stage with cornified epithelium with nucleus (circle) and without nucleus (smaller arrow) and neutrophils (larger arrow) (A, ×40; B, ×10). |
 | Fig. 4Diestrous stage with some cornified epithelium (large arrow) and numerous neutrophils (A, ×40; B, ×10). |
 | Fig. 5Normal estrous cycle of the control group. 1, prestrous; 2, estrous; 3, metastrous; 4, diestrous. |
 | Fig. 6Prolong diestrous stage of estrous cycle in the induced group. 1, prestrous; 2, estrous; 3, metastrous; 4, diestrous. |
 | Fig. 7Recovery of estrous cycle in the treatment group. 1, prestrous; 2, estrous; 3, metastrous; 4, diestrous. |
 | Fig. 8Recovery of estrous cycle in the referral group. 1, prestrous; 2, estrous; 3, metastrous; 4, diestrous. |
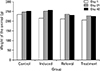 | Fig. 9Weight of the different groups of animals. Control, induced (polycystic ovarian syndrome), treatment (drug), and referral (metformin). |
 | Fig. 10Weight of the right and left ovary in different groups of animals. Control, induced (polycystic ovarian syndrome), treatment (drug), and referral (metformin) |
Acknowledgements
Authors thanks JKK Munirajah Medical Research Foundations College of Pharmacy, Tamil Nadu, Management of SLIMS Puducherry for their support and also acknowledge Dr. Preamaraja, Mr.Ayyanar@Muthu Attender, Mrs. Bakaiyalakshmi housekeeping for their kindly help and support last but not the least Mrs. V. Tamilselvi, M.Sc. statistician.
References
1. Reame N, Sauder SE, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J Clin Endocrinol Metab. 1984; 59:328–337.
2. Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981; 109:376–385.
3. Hayes FJ, Hall JE, Boepple PA, Crowley WF Jr. Clinical review 96: differential control of gonadotropin secretion in the human: endocrine role of inhibin. J Clin Endocrinol Metab. 1998; 83:1835–1841.
4. Roe AH, Dokras A. The diagnosis of polycystic ovary syndrome in adolescents. Rev Obstet Gynecol. 2011; 4:45–51.
5. Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome. The Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006; 91:781–785.
6. Hirsutism and polycystic ovary syndrome (PCOS) [Internet]. Burmingham, AL: American Society for Reproductive Medicine;2016. cited 2018 Sep 10. Available from: https://www.reproductivefacts.org/globalassets/rf/news-and-publications/bookletsfact-sheets/english-fact-sheets-and-info-booklets/booklet_hirsutism_and_pcos.pdf.
7. Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013; 78:734–740.
8. Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014; 32:183–193.
9. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012; 7:e35538.
10. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007; 80:84–97.
11. Rojas-Sandoval J, Acevedo-Rodriguez P. Cynodon dactylon (Bermuda grass) [Internet]. Wallingford: CAB International;2018. cited 2018 Sep 10. Available from: https://www.cabi.org/isc/datasheet/17463.
12. Kaup SR, Arunkumar N, Bernhardt LK, Vasavi RG, Shetty SS, Pai SR, Arunkumar B. Antihyperlipedemic activity of Cynodon dactylon extract in high-cholesterol diet fed Wistar rats. Genomic Med Biomark Health Sci. 2011; 3:98–102.
13. Kanimozhi DM, Bai RR. In vitro anticancer activity of ethanolic extract of Cynodon dactylon against HT-29 cell line. Int J Curr Sci. 2013; 5:74–81.
14. Karthik D, Ravikumar S. A study on the protective effect of Cynodon dactylon leaves extract in diabetic rats. Biomed Environ Sci. 2011; 24:190–199.
15. Ramesh H. Preclinical evaluation of protective effect of Cynodon dactylon pers on experimentally induced gastric mucosal damage. Res Rev J Med Health Sci. 2013; 2:89–93.
16. Devi HC, Moirangthem RS, Vikram SS, Devi KS, Devi KP, Rita S. Hepatoprotective activity of aqueous extract of Cynodon dactylon on paracetamol induced hepatotoxic albino rats. Sch J Appl Med Sci. 2017; 5:199–204.
17. Pandey K, Singh CS, Prasad RK, Singh AK, Mishra MK. Studies of anti-microbial activity using leaf extract of Cynodon dactylon. Pharm Lett. 2016; 8:325–330.
18. Sharma D. Study of antimicrobial activity of Cynodon dactylon. Res Rev J Microbiol Biotechnol. 2016; 26–31.
19. Pashaie B, Hobbenaghi R, Malekinejad H. Anti-atherosclerotic effect of Cynodon dactylon extract on experimentally induced hypercholesterolemia in rats. Vet Res Forum. 2017; 8:185–193.
20. Part 5: Preparation, reading and reporting of vaginal smears [Internet]. Paris: Organisation for Economic Cooperation and Development;2018. cited 2018 Aug 10. Available from: http://www.oecd.org/chemicalsafety/testing/40581357.pdf.
21. Reddy PS, Begum N, Mutha S, Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pac J Reprod. 2016; 5:116–122.
22. Vani , Gopalan DH, Manikandan S, Vijayakumar V. Letrozole and fructose-induced polycystic ovaries in the rat: a novel model exhibiting both ovarian and metabolic characteristics for polycystic ovary syndrome in rat. Int J Pharm Sci Res. 2018; 9:2238–2243.
23. Jahan S, Abid A, Khalid S, Afsar T, Qurat-Ul-Ain , Shaheen G, Almajwal A, Razak S. Therapeutic potentials of quercetin in management of polycystic ovarian syndrome using letrozole induced rat model: a histological and a biochemical study. J Ovarian Res. 2018; 11:26.
24. Karateke A, Dokuyucu R, Dogan H, Ozgur T, Tas ZA, Tutuk O, Agturk G, Tumer C. Investigation of therapeutic effects of erdosteine on polycystic ovary syndrome in a rat model. Med Princ Pract. 2018; 27:515–522.
25. Hong Y, Yin Y, Tan Y, Hong K, Jiang F, Wang Y. Effect of quercetin on biochemical parameters in letrozole-induced polycystic ovary syndrome in rats. Trop J Pharm Res. 2018; 17:1783–1788.
26. Basheer M, Rai S, Ghosh H, Ahmad Hajam Y. Protective role of seed extract of Tephrosia purpurea in letrozole induced polycystic ovary syndrome in Wistar rats. J Biol Sci. 2018; 18:458–467.
27. Abdollahifar MA, Azad N, Sajadi E, Shams Mofarahe Z, Zare F, Moradi A, Rezaee F, Gholamin M, Abdi S. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat Cell Biol. 2019; 52:196–203.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download