Abstract
The thymus is a lymphoepithelial organ, and its morphometry is commonly utilized for surveillance of the immunological status of fetus and neonates. Many studies showed that fetal thymus size is used as a prognostic indicator for pregnancy-related disorders such as eclampsia, preterm labor, and gestational diabetes. The study aims to establish reference ranges of the normal fetal thymus size between 12 and 40 weeks of gestational age (GA). The study was conducted on 89 fetuses. They were dissected to capture the morphometry of thymus: transverse diameter, perimeter, and weight. Considering these parameters were dependent variables of GA and gestational weight (GW). Their relationship was studied by a multiple regression model. The best fit models in predicting thymic dimensions as a function of GA and GW were determined using regression analysis. Mean transverse diameter, perimeter, and thymus weight was 33.45±2.91 mm, 125.72±55.4 mm, and 3.078±3.06 g, respectively. They were increased throughout pregnancy as GA and GW advanced. The regression equation for a transverse diameter of the thymus as a function of GA was (0.303×GA-4.885, R2=0.8196) and for the perimeter of the thymus was (1.0212×GA-15.24, R2=0.8666). Reference ranges and baseline data of the normal fetal thymic dimensions between 12 and 40 weeks of GA have been established.
Evaluating organ measurements against the known standard is very helpful for perinatal pathology. Major organs such as liver, lung, kidney, and adrenal gland have steady growth and least affected in immunological and nutritional challenges after 20 weeks except for thymus [1]. The thymus is a bilobed primary lymphoid organ. It has a significant role in the differentiation, selection, and maturation of T-cell lymphocytes [2]. The development of thymus begins at the 5th week of gestation from the third pharyngeal pouch (ventral part), and lymphocytes start to form in the thymus during the ninth week of gestation [3]. The thymus grows and enlarges continuously between the prenatal period and puberty. The thymus has an active response to systemic fetal inflammation during the prenatal period. The stress involution might appear during the prenatal phase in response to different forms of acute stress stimuli such as infection, trauma, sepsis, and malnutrition [4]. Endogenous corticosteroids and systemic inflammatory mediators trigger it in response to the above conditions [5,6,7]. These conditions are also leading causes of intrauterine growth retardation (IUGR) of a fetus. Further, IUGR predisposes risk of perinatal morbidity and mortality [8]. Such morbid conditions may alter the thymus size and cannot be used as baseline data, and further cannot be utilized for assessing the growth of the fetus in utero.
The current literature identifies fetal thymus size as the sensitive prognostic tool for pregnancy-related disorders e.g., eclampsia, preterm labor, chorioamnionitis with or without premature rupture of membrane, and gestational diabetes [69101112].
Assessment of thymic hypoplasia requires normal comparative data of thymic size respective to the gestational age (GA). Four sets of measurement were considered by most of the authors, e.g., transverse diameter, thymic perimeter, thymic index, and thymic-thoracic ratio. The data regarding these parameters were collected mostly by ultrasonography, and few were by magnetic resonance imaging [131415]. The empirical and baseline data of fetal thymuses were found highly variable due to the differing methodologies. Also, the difference in demographic parameters like racial or cultural or nutritional may affect the development of thymus in utero.
There is a paucity of data from dissected human fetuses for correlation. Majority of the authors considered only single parameters for their study in published literature either transverse diameter or perimeter. Thymus diameter measured by ultrasonography in 250 fetuses, and it was observed that diameter was not reliable after 28 weeks of gestation [1617].
This study considered the addition of thymus weight, transverse diameter, and perimeter to improve sensitivity and specificity as a prognostic indicator because of its asymmetric shape.
The cross-sectional study was done on the fetal thymus to derive baseline data and to establish the accurate reference value for all trimester from dissected fetal thymus and its association with GA or gestational weight (GW).
The ethics committee of the institute approved the study (TEC No. IEC/07.02.2008 and 46/RIMS&R/2015-16) and performed as per the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Formalin-fixed 140 fetuses from the museum of the Department of Anatomy of two medical colleges were selected. The age of the fetus was estimated by measuring crown-rump length, head circumference, abdominal circumference, foot length, and matched with GA documented in procurement record based on history and ultrasonographic findings of patients. The same determined age of fetuses further matched with charts of fetal morphometrics to avoid any error [17]. The fetuses having a weight below the 10th percentile for gestational age, macerated thymus congenital anomalies, placental, or umbilical cord anomalies and history of chronic maternal illness, eclampsia, diabetes, hypertension, and smoking were excluded from the study. The fetuses aged between 12 and 40 weeks with weight above the 10th percentile were included in 93 fetuses (Fig. 1A). For convenience, all fetuses were classified into seven groups as per GA at 4-week interval from 12 weeks to 40 weeks (group I to VII).
The thymus was dissected by a midline incision from the thyroid cartilage to below to xiphisternum and even extended up to umbilicus in some cases to get a clear view. The skin and superficial fascia have reflected both sides. The deep cervical fascia was incised at midline till sternum. The sternocleidomastoid muscles were reflected on the corresponding side. The upper end of the infrahyoid muscles was detached. The soft tissues and fat were removed to get a clear visualization of the fetal thymus as an oval bilobed structure.
Maximum transverse diameter, maximum length, and thickness were recorded by digital vernier caliper (sensitivity, 0.01 mm) in situ without detaching it from vascular bed (Fig. 1B). Then thymus was detached from its bed, especially from brachiocephalic veins. Both surfaces of the thymus were colored green with the help of soft brush and impression was made at graph paper. The perimeter of the fetal thymus was recorded by scan image of graph paper with the help of the ruler of Adobe Photoshop CS 5.0 (Fig. 1C).
Statistical analysis was performed with MS excel 2010 and SPSS version 22.0.0.0 for the Windows (IBM Corp., Armonk, NY, USA). The t test applied for intergroup variation, and ANOVA was used for all groups to compare the mean. The intraobserver agreement was measured with the help of Bland Altman plot. For studying the impact of the size of the fetus on the dimensions of the thymus, the ratio between the thymus perimeter and fetal anthropometric parameters were calculated. The fetal thymus perimeter increases as a function of GA and GW. For studying the association among them, the multiple regression was applied by using open least square (OLS) regression. For assessing the linear model, fitted value, the standardized residual value was calculated, and the residual plot and the scattered graph was traced between the fitted value and observed value for each multiple regression models for prediction and explanation considering P<0.05 significant (Figs. 2, 3).
Measurements of thymus size were obtained in 89 fetuses between 12 and 40 weeks gestation in which 37 were male fetuses and 52 were female fetuses. Mean age of fetuses was 27.56±8.24 weeks. The mean weight of the thymus was 3.783±1.05 g (Tables 1, 2). The intraobserver agreement was under the confidence limit of 95%.
The mean transverse diameter of the fetal thymus was 33.45±2.91 mm (Table 2). In fetuses of 12 to 16 weeks (group I) were having a transverse diameter from 2 to 3 mm (2.3±0.5 mm). In the next 4 weeks (group II) transverse diameter ranged from 4 to 13 mm (6.87±3.23 mm). In group III (20–24 weeks), the transverse diameter ranged from 6 to 18 mm (9.71±4.5 mm) and the same reached up to 24 mm (15±3.52) in the early third trimester (group IV, 24-28 weeks). During 28–32 weeks (group V) and 32–36 weeks (group VI), transverse diameter were 30–47 mm (42±6.96 mm) and 52–70 mm (64.1±6.46 mm), respectively. In group VII (36–40 weeks) fetuses, the transverse diameter of thymus fluctuated between 68 and 78 mm (75.7±5.46 mm). Transverse diameter of thymus has strong relationship with GA (0.303×GA-4.885, R2=0.8196) and GW (0.0024×GW+0.0843, R2=0.9259) (Tables 3, 4, Fig. 2).
The mean perimeter of the thymus was 125.72±55.4 mm (Table 1). It was ranged 7.7–14.4 mm (9.99±3.48 mm), 21–62 mm (32.2±14.33 mm), 46–73 mm (51.23±11.07 mm), 70–103 mm (78.46±20.63 mm), 124–163 mm (143.23±14.8 mm), 196–227 mm (221.9±16 mm), and 236–288 mm (263.48±21.7 mm) in group I to VII fetuses, respectively (Table 2). The parameter have a significant correlation with GA (1.0212×GA-15.24, R2=0.8666) and GW (0.0081×GW+1.6984, R2=0.9498) (Tables 3, 4, Fig. 2).
The mean thymus weight was 3.078±3.06 g (Table 2). The weight of thymus in group I to group VII varied from 0.1 to 0.2 g (0.13±0.05 g), 0.3-1 g (0.5±0.22 g), 1-2.5 g (1.78±0.48 g), 2.5–3 g (2.55±0.53 g), 3–5.5 g (4.3±1.75 g), 4.5–7.4 g (5.95±0.99 g), and 7–10 g (8.45±1.17 g), respectively. Like other parameters, it also has a strong correlation with GA (R2=0.844) and GW (R2=0.952). The weight of thymus has a strong association with perimeter and transverse diameter in the OLS multiple regression. The derived relation was predicted thymus weight (g)=0.44 (perimeter-transverse diameter)-0.32. The same could be predicted with GA and weight with thymus weight (g)=0.12×GA (weeks)+1.8×GW (kg)-1.86 (Tables 3, 4, Fig. 3).
The human thymus is a delicate organ which is under the influence of environmental factor, nutritional and immunological status. This study aimed to determine the perimeter and transverse diameter of the fetal thymus in normal human fetuses aged between 12 and 40 weeks. Secondarily we were able to develop a normative data of thymus size that could be utilized as a prognostic indicator of perinatal morbidity.
Thymic index, thymus volume, and transverse diameter computed in 212 full-term fetuses. The above parameters were 9.08±2.46, 13.22±3.82 cm3, and 2.37±0.32 cm, respectively [15]. The mean fetal thymic transverse diameter measured at 19th week was 12 mm and at 33rd week was 33 mm while at term was slightly higher than GA [18]. They have defined that growth pattern of the thymus was similar to other fetal organs like liver, kidney, colon-rectum, and thyroid [19202122]. Their findings were also similar as in group II and group V fetuses of this current study. Gur et al. [23] discussed the normal fetal thymic perimeter was 93–94 mm, and it was significantly higher than vitamin D deficiency fetuses 76–85 mm (P=0.04). In another literature, fetal thymic transverse diameter during the second and third trimester of uncomplicated singleton pregnancy was ranged 12 to 25 mm in 18–28 weeks and ranged 27 to 40 mm in 29–38 weeks. Our finding in the same age group was similar or slightly higher side. The disparity in the value might have arisen from the methodological difference. Gamez et al. [24] measured the transverse diameter was 24.22±6.7 mm (singleton pregnancy) and 23.9±6.3 mm (twin pregnancy) with P-value of 0.2 (insignificant difference). Another author conducted a study on 296 women to measure the maximum diameter of thymus and its relation with GA and biparietal diameter (BPD). They reported a significant correlation of diameter of thymus with GA (r=0.915, P<0.001) and BPD (r=0.916, P<0.001) and given regression equation 2.476×GA-0.019×GA2-25.904 and 0.522×BPD-8.245 in millimeter [25]. This study observed the mean transverse diameter of the thymus as 33.45 mm and found effective associations with GA and GW.
In addition to the above finding Gamez et al. [24] also evaluated the thymic perimeters were 67.9±19.9 mm (singleton pregnancy) and 66.3±20 mm (twin pregnancy) without any significant difference (P=0.3). Zalel et al. [22] conducted an ultrasonic evaluation of thymus in 403 fetuses in Israeli population and found mean perimeter at 14 week of gestation was 22.42 mm (17.97–26.87 mm), and at the 38th week of gestation, it became 128.38 mm (121.08–135.66 mm). According to these authors, the size of the thymus has a strong correlation with age (r=0.965) and thymus perimeter (mm)=4.41×GA-39.39 by regression analysis with 90% prediction limit [22]. The transverse diameter of the fetal thymus estimated in 98 fetuses by ultrasonography, and its median value was 27.5 mm, and the range was 22.5-31.4 mm [26]. The perimeter of fetal thymus measured and reported a strong association with GA and BPD [25]. In our study, the perimeter of human fetal thymuses was 125.72 mm. It has a significant relationship with GA and GW. The difference between ultrasonography and our observations existed. It might be possible due to equivalent echogenicity of the thymus to surrounding lung tissue, which compromises the accuracy due to the difficulty to localize and delineate fetal thymus for measurements by ultrasound.
The weight of thymus measured in 250 fetuses aged from GA 12–26 weeks without considering sex, racial, and ethnicity variation, which was varied from 0.01 to 2.04 g [27]. The weight of thymus evaluated in 673 formalin-fixed fetuses (13–42 weeks of gestation), and they found the range from 0.09 to 22.34 g. They also analyzed the 5th and 95th percentile value. The 5th percentile of thymic weight was ranged from 0.04 to 11.75 g, and 95th percentile value ranged from 0.20 to 32.93 g for the aforementioned GA [28]. In our study, the mean weight of thymus was 3.783 g and range value was 0.10 to 10 g for GA from 12 to 40 weeks.
Most of the authors have taken different parameters to assess the growth of the thymus in the fetus by ultrasonography. Localization of fetal thymus in the early weeks of gestation is not so easy and recording the transverse diameter, perimeter, or surface area are varied according to the mobility of fetus, techniques, and machines. Analyzing the transverse diameter and perimeter of the thymus are two-dimensional analysis, but for healthier analysis, we should consider three-dimensional analysis like weight or volume of the thymus. The previous literature did not provide a relation of thymus weight with transverse diameter and perimeter or surface area by ultrasonography. Most of the literature showed the correlation of parameter of thymus or thymic index with GA and GW. In our study, we have taken thymus weight, transverse diameter, and perimeter along with GA and GW and computed relation among them by regression analysis. In vivo measurement of thymic parameters in fetuses has better accuracy than ultrasonographic measurements because of easy visualization and high intraobserver and interobserver correlation coefficient with 95% confidence limit. We have introduced the computation of thymus weight from transverse diameter and perimeter (Table 3).
In conclusion, the current study provides the relation between thymic size with GA and GW, which can be utilized for developing nomogram and for assessment of fetal growth in utero. This relation could be used in mortality meet by the clinician to assess fetal progress or deterioration during perinatal disorder or perinatal pathologist to compare with the normal and possible cause of death to some extent. It is a well-known fact that maternal biochemical parameters, such as C-reactive protein, procalcitonin, and leukocytosis, are inadequate, and monitoring of thymus would be of good prognostic value in intrauterine disorders. Thymic parameters will help to study the growth pattern and immunological development of fetuses and any value beyond 95th or 5th percentile considered abnormal will be the sign of fetal stress and needs to investigate the possible reason of fetal distress like preterm labor or premature rupture of membrane, gestational diabetes or history of smoking. The size of the thymus is utilized as a prenatal marker for the fetal immune-endocrine response to malnutrition. These data cannot be used for postnatal screening, and this limitation gives future scope for further study in postneonatal and pediatric age groups. Further, the correlation between the thymic size and the percentage of T cells, complement and thymosin levels in cord blood could be studied.
Acknowledgements
We are thankful to Faculties of Dept of Anatomy, JNMC Aligarh and UPUMS Saifai for their immense support.
References
1. Man J, Hutchinson JC, Ashworth M, Jeffrey I, Heazell AE, Sebire NJ. Organ weights and ratios for postmortem identification of fetal growth restriction: utility and confounding factors. Ultrasound Obstet Gynecol. 2016; 48:585–590. PMID: 27781326.
2. Ciofani M, Zúñiga-Pflücker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007; 23:463–493. PMID: 17506693.
3. Jeppesen DL, Hasselbalch H, Nielsen SD, Sørensen TU, Ersbøll AK, Valerius NH, Heilmann C. Thymic size in preterm neonates: a sonographic study. Acta Paediatr. 2003; 92:817–822. PMID: 12892161.
4. Toti P, De Felice C, Stumpo M, Schürfeld K, Di Leo L, Vatti R, Bianciardi G, Buonocore G, Seemayer TA, Luzi P. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000; 31:1121–1128. PMID: 11014581.
5. Pagenkemper M, Diemert A. Monitoring fetal immune development in human pregnancies: current concepts and future goals. J Reprod Immunol. 2014; 104-105:49–53. PMID: 25124491.
6. Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D'Addario V, Loverro G. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006; 194:153–159. PMID: 16389025.
7. Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003; 112:1310–1312. PMID: 14597756.
8. Cox P, Marton T. Pathological assessment of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009; 23:751–764. PMID: 19854107.
9. Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, Achiron R. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 2007; 29:639–643. PMID: 17471450.
10. Cromi A, Ghezzi F, Raffaelli R, Bergamini V, Siesto G, Bolis P. Ultrasonographic measurement of thymus size in IUGR fetuses: a marker of the fetal immunoendocrine response to malnutrition. Ultrasound Obstet Gynecol. 2009; 33:421–426. PMID: 19306477.
11. Mohamed N, Eviston DP, Quinton AE, Benzie RJ, Kirby AC, Peek MJ, Nanan RK. Smaller fetal thymuses in pre-eclampsia: a prospective cross-sectional study. Ultrasound Obstet Gynecol. 2011; 37:410–415. PMID: 21308839.
12. Olearo E, Oberto M, Oggè G, Botta G, Pace C, Gaglioti P, Todros T. Thymic volume in healthy, small for gestational age and growth restricted fetuses. Prenat Diagn. 2012; 32:662–667. PMID: 22544629.
13. Aaby P, Marx C, Trautner S, Rudaa D, Hasselbalch H, Jensen H, Lisse I. Thymus size at birth is associated with infant mortality: a community study from Guinea-Bissau. Acta Paediatr. 2002; 91:698–703. PMID: 12162605.
14. Park HY, Hertz-Picciotto I, Petrik J, Palkovicova L, Kocan A, Trnovec T. Prenatal PCB exposure and thymus size at birth in neonates in Eastern Slovakia. Environ Health Perspect. 2008; 116:104–109. PMID: 18197307.
15. Varga I, Uhrinova A, Toth F, Mistinova J. Assessment of the thymic morphometry using ultrasound in full-term newborns. Surg Radiol Anat. 2011; 33:689–695. PMID: 21442251.
16. Acharya P, Acharya A. Evaluation of applicability of standard growth curves to Indian women by fetal biometry. J S Asian Fed Obstet Gynecol. 2009; 1:55–61.
17. Muñoz-Chápuli M, Gámez F, Bravo C, Ortiz L, Pérez R, De León-Luis JA. The thy-box for sonographic assessment of the fetal thymus: nomogram and review of the literature. J Ultrasound Med. 2015; 34:853–858. PMID: 25911720.
18. Cho JY, Min JY, Lee YH, McCrindle B, Hornberger LK, Yoo SJ. Diameter of the normal fetal thymus on ultrasound. Ultrasound Obstet Gynecol. 2007; 29:634–638. PMID: 17385216.
19. Murao F, Takamori H, Hata K, Hata T, Kitao M. Fetal liver measurements by ultrasonography. Int J Gynaecol Obstet. 1987; 25:381–385. PMID: 2889632.
20. Ho SS, Metreweli C. Normal fetal thyroid volume. Ultrasound Obstet Gynecol. 1998; 11:118–122. PMID: 9549838.
21. Gloor JM, Breckle RJ, Gehrking WC, Rosenquist RG, Mulholland TA, Bergstralh EJ, Ramin KD, Ogburn PL Jr. Fetal renal growth evaluated by prenatal ultrasound examination. Mayo Clin Proc. 1997; 72:124–129. PMID: 9033544.
22. Zalel Y, Perlitz Y, Gamzu R, Peleg D, Ben-Ami M. In-utero development of the fetal colon and rectum: sonographic evaluation. Ultrasound Obstet Gynecol. 2003; 21:161–164. PMID: 12601839.
23. Gur EB, Gur MS, Ince O, Kasap E, Genc M, Tatar S, Bugday S, Turan GA, Guclu S. Vitamin D deficiency in pregnancy may affect fetal thymus development. Ginekol Pol. 2016; 87:378–383. PMID: 27304655.
24. Gamez F, De Leon-Luis J, Pintado P, Perez R, Robinson JN, Antolin E, Ortiz-Quintana L, Santolaya-Forgas J. Fetal thymus size in uncomplicated twin and singleton pregnancies. Ultrasound Obstet Gynecol. 2010; 36:302–307. PMID: 20131331.
25. Tangshewinsirikul C, Panburana P. Sonographic measurement of fetal thymus size in uncomplicated singleton pregnancies. J Clin Ultrasound. 2017; 45:150–159. PMID: 27862004.
26. Musilova I, Hornychova H, Kostal M, Jacobsson B, Kacerovsky M. Ultrasound measurement of the transverse diameter of the fetal thymus in pregnancies complicated by the preterm prelabor rupture of membranes. J Clin Ultrasound. 2013; 41:283–289. PMID: 23505029.
27. Hansen K, Sung CJ, Huang C, Pinar H, Singer DB, Oyer CE. Reference values for second trimester fetal and neonatal organ weights and measurements. Pediatr Dev Pathol. 2003; 6:160–167. PMID: 12548377.
28. Guihard-Costa AM, Ménez F, Delezoide AL. Organ weights in human fetuses after formalin fixation: standards by gestational age and body weight. Pediatr Dev Pathol. 2002; 5:559–578. PMID: 12399830.
Fig. 1
(A) Methodology for study of growth pattern of thymus. (B) Measurement of Transverse diameter in dissected thymus by Digital Vernier Caliper. (C) Measurement of perimeter and area by taking impression on graph paper.
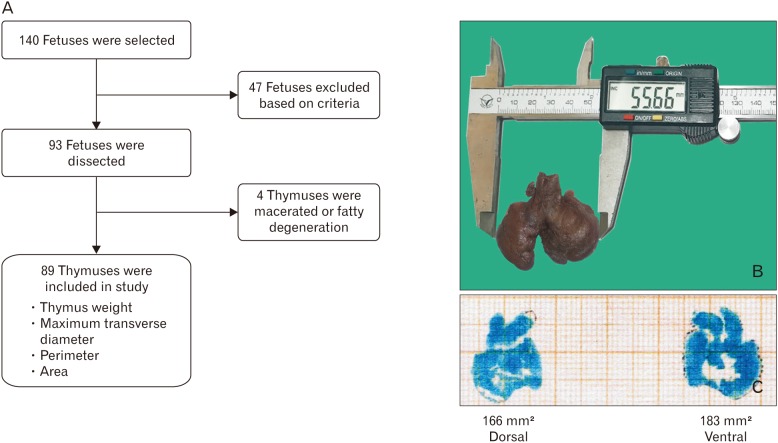
Fig. 2
Multiple regression analysis of transverse diameter with gestational age and weight (A) and perimeter with gestational age and weight (B).
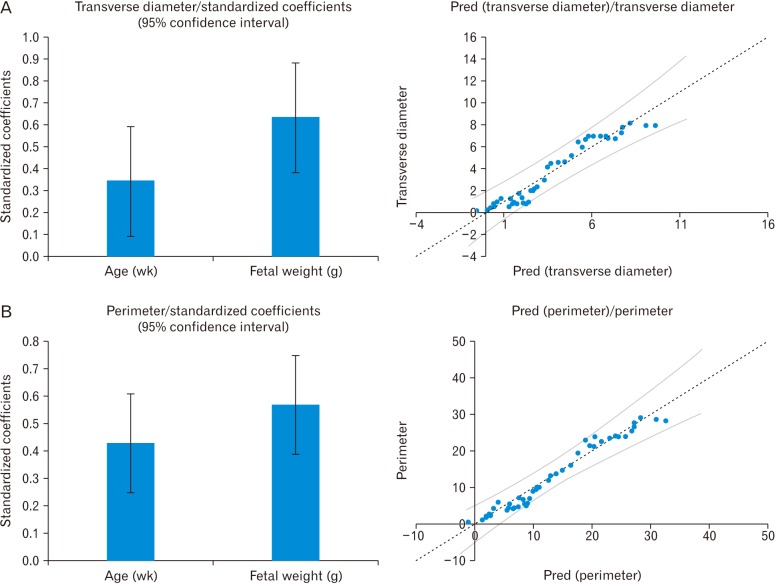
Fig. 3
Multiple regression analysis of thymus weight with gestational age and weight (A) and thymus weight with transverse diameter and perimeter of thymus (B).
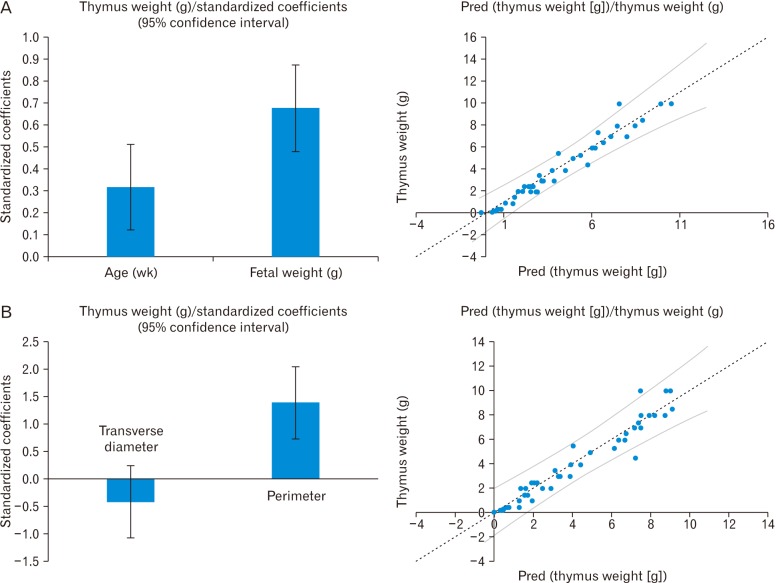
Table 1
Distribution of fetuses

| Distribution | Range | Mean±SD | Correlation |
|---|---|---|---|
| No. of fetuses | 89 (male 37, female 52) | ||
| Gestational age (wk) | 12–40 | 27.56±8.25 | |
| Gestational weight (g) | 52–3,097 | 1,341.25±1,151.02 | P=1.89E-25 |
| R2=0.9075 with age |
Table 2
Parameters of thymuses
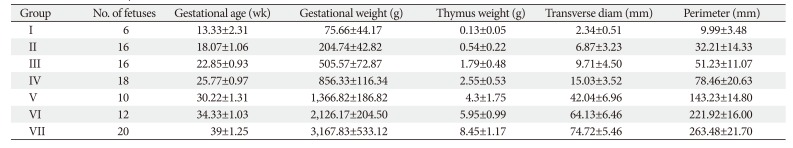
Table 3
Correlation of thymic parameters with gestational age and weight
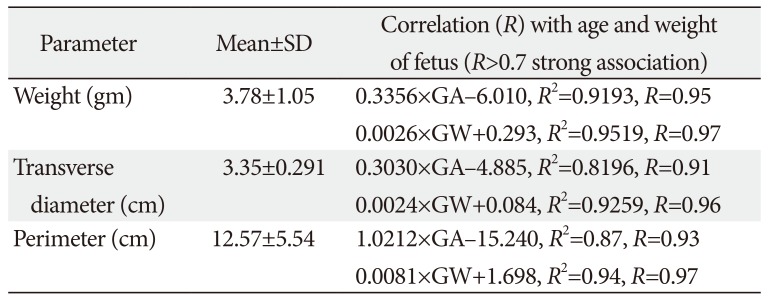
Table 4
Multiple regression of thymic parameters with gestational age and weight





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download