Abstract
Frontolateral craniotomy procedures have advanced from conventional craniotomy to mini-craniotomy, and to contemporary keyhole surgery. In this context, it is important for the neurosurgeon to precisely locate the pterion. The distance of the pterion center from midpoint of zygomatic arch and posterolateral margin of frontozygomatic suture was studied bilaterally in 50 whole adult skulls in Indian ethnic group. The depth of optic canal and sphenoid ridge from the pterion was recorded bilaterally in fifty cut adult skulls and fifteen three-dimensional computed tomography scans. The suture length, thickness, and morphology were studied. The data were analyzed using SPSS software, two-tailed Student's t test, binary logistic regression and receiver operating characteristic curve for sexual dimorphism. The pterion center was located at a mean distance of 37.02 mm above the midpoint of zygomatic arch, 28.20 mm behind the posterolateral margin of frontozygomatic suture, 42.73 mm lateral to the optic canal and 10.59 mm from the sphenoid ridge. The location did not exhibit sexual dimorphism. In 20% cases the pterion center was 40 mm or more above the midpoint of the zygomatic arch and in 5% cases 35 mm or more posterior to the posterolateral margin of frontozygomatic suture. The mean suture length was 10±3 mm. The mean thickness at the center of the pterion was 3.52±1.45 mm. The commonest variety was sphenoparietal followed by frontotemporal, epipteric, and stellate types. A thorough knowledge of these dimensions has innumerable neurosurgical implications in resection of sellar, parasellar, and paraclinoid tumors and circulatory aneurysms.
The pterion is an area of confluence of frontal, parietal, squamous temporal and greater wing of sphenoid bones in the Norma lateralis of the skull [1]. It was first classified by Broca [2] into pterion en H, pterion retourne and pterion en K representing the sphenoparietal, frontotemporal and stellate varieties. The epipteric variety was later included by Murphy [3] who reported 16 combinations.
Frontolateral craniotomy procedures have advanced over the years from conventional craniotomy to mini-craniotomy to contemporary keyhole surgery [4]. The trans-Sylvian approach through the pterion facilitates resection of tumors of sellar, parasellar, and paraclinoid regions with minimum craniotomy [5]. It is also the preferred approach for aneurysm clipping and resection of optic nerve meningiomas [678].
The pterion is also the site of trephination for extradural hematoma. A circle of 1-cm radius with the midpoint of the pterion as the center overlaps the anterior branch of middle meningeal artery in 68% cases and in 32% cases the vessel is located posteriorly [9]. In the epipteric variety, the anterior most point of junction of the four bones may be mistaken to be the center of the pterion (CP) and anterior drilling can result in orbital penetration while a posterior drilling results in neurosurgical complications [10]. In this context, it is important for the neurosurgeon to precisely identify the pterion center and understand its morphology.
While most studies focus on the external location and morphology of the pterion [1112], our study analyses the depth of specific intracranial landmarks from the pterion center considering them to be more important from neurosurgeon's perspective. The optic canal (OC) and the sphenoid ridge (SR) are two such intracranial landmarks of profound significance to neurosurgeons owing to their proximity to diverse neoplasms and aneurysms. The depth of these landmarks from the pterion center is analyzed both in dry skulls and three-dimensional computed tomography (CT) scans. The pterion morphology and its distance from specific extracranial landmarks are also studied in Indian ethnic group. The pterion location is reported to be higher in males than females [1112]. However, in this study no such variation was observed. The ethnic variation in pterion location is also reviewed and summarized.
The pterion location and morphology were studied in 100 skulls (66 male and 34 female), aged between 25 to 68 years, in the Department of Anatomy, Yenepoya Medical College, Mangalore, India following clearance from the institutional ethical clearance committee. The skulls were procured from a cemetery in Mangalore, Karnataka state, India. Only completely ossified adult human skulls without any deformities were selected. All measurements were done using a sliding digital caliper (Lianying 0005, Zhejiang, China) graduated to the last 0.01 mm.
A circle with the smallest radius connecting all the four bones involved in the formation of the pterion was marked as shown in Fig. 1. The center of this circle was marked as the CP. The midpoint of the zygomatic arch (Z) and the posterolateral margin of the frontozygomatic suture (S) were marked. The distance of the pterion center from these points was measured in 50 whole skulls as shown in Fig. 2. The pterion center located equal to or more than 40 mm above the midpoint of zygomatic arch was considered as high location and equal to or more than 35 mm posterior to the frontozygomatic suture was considered as posterior location. The suture length was measured as shown in Fig. 3.
Fifty skulls were cut at a plane above the inion and the supraorbital ridge. The point corresponding to the CP was marked on the internal aspect in these skulls as the internal location of the pterion (IP) after holding the skulls in Frankfurt plane [13]. The distance of this point from the lateral margin of the OC and the lateral margin of the SR was measured in the fifty cut skulls bilaterally as shown in Fig. 4. The skull thickness at the CP was also measured. The pterion morphology was classified in accordance with Murphy's classification into frontotemporal, sphenoparietal, stellate, and epipteric varieties [3] as shown in Fig. 5. The depth of the OC and the SR from the IP was measured bilaterally in 15 three-dimensional CT scans as shown in Fig. 6.
The data obtained were analyzed statistically with SPSS version 20.0 (IBM Corp., Armonk, NY, USA), two-tailed Student's t test (P<0.05), binary logistic regression and receiver operating characteristic curve for sexual dimorphism. Systematic and casual error and intra-observer reliability were checked using paired t test and Dahlberg's index.
Table 1 illustrates the mean distance of the pterion center from midpoint of zygomatic arch and posterolateral margin of frontozygomatic suture in males and females. The pterion center was located at a mean distance of 37.02 mm above the midpoint of zygomatic arch and 28.20 mm behind the posterolateral margin of frontozygomatic suture. In 20% cases it was located 40 mm or more above the midpoint of the zygomatic arch, the highest location being 50 mm and in 5% cases 35 mm or more posterior to the posterolateral margin of frontozygomatic suture, the most posterior location being 39 mm. None of the external dimensions exhibited significant sexual dimorphism except the distance of the pterion center from the posterolateral margin of frontozygomatic suture on the right side (P=0.013).
Table 2 illustrates the mean depth of OC and the SR from the pterion center in dry cut skulls bilaterally in males and females. The OC was located at a mean depth of 42.73 mm in dry skulls and 44.68 mm in 3-dimensional CT scans. The SR was located at a mean depth of 10.59 mm in dry skulls and 15.35 mm in CT scans. This difference between dry skulls and CT scans is statistically significant (P=0.006 for OC and P<0.001 for SR). None of the dimensions show significant sexual dimorphism.
The sutural morphology of the pterion was studied in accordance with Murphy's classification and it was observed that sphenoparietal was the commonest (83%) followed by frontotemporal (10%), epipteric (6%), and stellate (1%) varieties. No specific association was observed between the type of the pterion and side of the skull or sex of the individual. The frequency of each type in either sex is summarized in Table 3.
The mean suture length pooling the sphenoparietal and frontotemporal types was observed to be 11.85±5.21 mm and 12.23±4.78 mm on the right and left sides respectively in males. The mean length was 12.81±6.26 mm and 12.37±6.21 mm on the right and left sides respectively in females. The mean thickness at the CP was 3.52±1.45 mm.
A meta-analysis on the worldwide incidence of all brain tumors reveals an incidence of 10.82 tumors per 100,000 person-years [14]. The basic principle in brain tumor surgery is to achieve maximum tumor resection with minimum functional impairment using minimally invasive techniques [4]. In this context, the recent advances in frontolateral craniotomy procedures such as keyhole surgeries assume significance in surgical management of diverse neoplasms [5]. The pterional craniotomy when introduced by Heuer and Dandy in the year 1920 involved excising a larger segment of skull [15]. The area of craniotomy flap has reduced over the years to minipterional craniotomy and keyhole surgery [16]. This implies that the neurosurgeon must locate the pterion center precisely as drilling at an improper site can result in orbital penetration or neurosurgical complications [17].
In the present study in Indian ethnic group the pterion center was located at a mean distance of 37.02 mm above the midpoint of zygomatic arch, 28.20 mm behind the posterolateral margin of frontozygomatic suture, 42.73 mm lateral to the OC, and 10.59 mm from the SR in dry skulls. Table 4 depicts the ethnic variation in the pterion position as reported by diverse authors. Such a comparison reveals that the pterion location varies by only a few millimeters in diverse ethnic groups and it is difficult to attribute this variation entirely to ethnicity. Moreover, in many studies such as the study by Oguz et al. [18] in 26 Turkish dry skulls and Adejuwon et al. [12] in 37 adult Nigerian skulls the sample size is too small to apply the results to an entire ethnic population. We believe that to conclusively demonstrate ethnic variation in the pterion position, the sample size must be much larger. The variation in position can also be due to sex which needs to be differentiated from ethnic variation as pointed by some authors. Mwachaka et al. [11] in their study in 90 adult Kenyan skulls and Adejuwon et al. [12] in 37 adult Nigerian skulls reported that males had significantly higher pterion position when compared to females. In our study, no such intersex variation was observed in the pterion height. In most studies the sample size is less than 100, hence too small to conclusively attribute the pterion position variation to either ethnicity or sex of the individual.
Most conventional studies focus on the pterion distance from the midpoint of zygomatic arch and posterolateral margin of frontozygomatic suture. Aksu et al. [19] studied the pterion distance from additional landmarks such as the zygomatic angle, the mastoid process and external acoustic meatus. In their study involving 128 adult west Anatolian skulls, the mean distance between the foremost point of the pterion and the anterior edge of the lateral wall of the orbit was also recorded [19]. Ma et al. [9] also described a method to locate the pterion center using only frontozygomatic suture as a surface landmark. They reported that the CP was a mean of 11±4 mm above and 26±4 mm behind the posterolateral margin of the frontozygomatic suture and that it was applicable to both sides and sex [9]. The ossification of parietal bone begins at the parietal eminence and spreads like spokes of a wheel as a result of which the bony ends remain unossified at birth forming fontanelle. The anterolateral fontanelle ossifies in the first few months and forms the pterion [21]. Aydin et al. [22] defined the pterion position in neonates by studying the location of 35 neonatal anterolateral fontanelle. They reported that to mark the pterion in neonates first a vertical line should be drawn 1.5 cm behind the orbital rim and then a horizontal line 1 cm above the zygomatic arch. The anterolateral fontanelle is marked by a square of 1 cm2 area in the posterosuperior region of these lines [22].
The tumor size and location are two significant factors determining the area of craniotomy. A keyhole craniotomy is preferred for minute aneurysm clipping procedures and small tumors along the Sylvian fissure while mini-craniotomy and larger craniotomies are reserved for larger tumors [57]. Myriad tumors lie in close proximity to OC and SR. These include tumors of sellar, parasellar, paraclinoid regions, posterior inferior frontal lobe, midbrain, temporal lobe, amygdala, hippocampus, cavernous sinus hemangiomas, optic and olfactory meningiomas and lipomas [56]. While there are innumerable studies on surface landmarks, there are few on these deeper dimensions which are more important from neurosurgical perspective. In this study, these dimensions are analyzed both in dry skulls and 3-dimensional CT scans. We observed that the depth of OC and SR is 4-5 mm more in CT scans which can be attributed to the presence of brain and cerebrospinal fluid (CSF) filled ventricles and subarachnoid space in vivo. The dimensions also depend on cranial index which differs in diverse ethnic groups [23].
Sphenoparietal is the most common type in Homo sapiens and frontotemporal is the most common type in primates. During evolution, the anterosuperior portion of squamous temporal bone gets detached and becomes incorporated into posterosuperior part of greater wing of sphenoid thus transforming the frontotemporal pattern in primates to sphenoparietal in Homo. If the detached portion remains as a sutural bone, the epipteric variety is formed [24]. In our study, sphenoparietal was the most common type followed by frontotemporal, epipteric and stellate types. Similar results were observed by Ilknur et al. [20] in 44 Anatolian skulls. Matsumura et al. [25] also studied the pterion morphology and formation in 614 Japanese skulls and reported that the pterion formation occurs in two phases. The first phase is before closure of anterolateral fontanelle and the second after 40 years [25]. The sutural morphology of the pterion in diverse ethnic groups is summarized in Table 5.
The mean suture length pooling the sphenoparietal and frontotemporal types was observed to be 11.85±5.21 mm and 12.23±4.78 mm on the right and left sides respectively in males. The mean length was 12.81±6.26 mm and 12.37±6.21 mm on the right and left sides respectively in females. In another study in Thai population the mean length of sphenoparietal suture was 9.04±6.04 mm and frontotemporal was 11.60±4.48 mm [13]. In a study by Murphy [3] in aborigines the mean length of sphenoparietal suture was 6.5±3.6 mm and the frontotemporal suture was 11.2±4.2 mm. The mean thickness at the CP observed in our study was 3.52±1.45 mm. The skull thickness at the CP, reported in other studies includes 5.13±1.67 mm in Thai skulls [13], 3.9 to 4.1 mm in Turks [18], and 3.19±0.85 mm in Korean skulls [26]. In another study, it was reported that the parietal bone thickness was more in blacks than whites and in males than females [27]. These values are useful for neurosurgeons for internal and external fixation during neurosurgical procedures.
Recent advances in pterional approach facilitate resection of myriad tumors and circulatory aneurysms with minimum craniotomy. In our study, the pterion position did not exhibit significant sexual dimorphism. Meanwhile, some researchers report that the pterion position is significantly higher in males than in females. The pterion position exhibits mild ethnic variation. No intersex variation was observed in the depth of OC and SR from the pterion, either. However, ethnic variation is likely as the breadth of skull and the cranial index varies in diverse ethnic groups. Moreover, there was significant difference in depth between dry skulls and 3-dimensional CT scans with the CT scan values being 4-5 mm higher. This can be attributed to the presence of brain and CSF filled ventricles and subarachnoid space in vivo.
Literature review reveals that humans sphenoparietal is the most common type and stellate the least common. The epipteric and frontotemporal varieties occur with varying frequencies. In case of an epipteric variety, caution must be observed, as the anterior most point of junction of the four bones may be mistaken to be the pterion center resulting in orbital penetration. The thickness of the skull bones shows ethnic variation and is more in males. The location of the middle meningeal artery with respect to the pterion was not studied and this is a limitation of our study. This study defines the location of the pterion center with respect to specific external and clinically relevant intracranial landmarks both in dry skulls and three-dimensional CT scans. A thorough knowledge of these dimensions has innumerable neurosurgical implications in resection of myriad intracranial tumors and circulatory aneurysms.
Figures and Tables
 | Fig. 1The technique of marking the center of the pterion externally. CP marks the location of the pterion. Type, sphenoparietal. CP, center of the pterion; F, frontal bone; P, parietal bone; S, sphenoid bone; T, temporal bone. |
 | Fig. 2The location of the pterion as linear distances from specific landmarks. CP, center of the pterion; CPS, distance from the center of the pterion to the posterolateral margin of the frontozygomatic suture; CPZ, distance from the center of the pterion to the midpoint of the zygoma; F, frontal bone; FZ, the frontal process of the zygomatic bone with the fronto-zygomatic suture intervening; S, posterolateral margin of the frontozygomatic suture; Z, midpoint of the zygoma. |
 | Fig. 3The technique of measurement of the length of the suture. Type of pterion, sphenoparietal. C, distance between the points A and B and marks the length of the suture; F, frontal bone; P, parietal bone; S, sphenoid bone; T, temporal bone. |
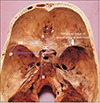 | Fig. 4The measurement of depth optic canal and the sphenoid ridge from the pterion. AB is a vertical line drawn at the lateral margin of the optic canal. IP-L is the horizontal distance from the internal location of the pterion to the lateral margin of the optic canal. IP-SR is the linear distance from the internal location of the pterion to the lateral end of the sphenoid ridge. IP, internal location of the pterion; OC, optic canal; SR, a point on the lateral end of the sphenoid ridge. |
 | Fig. 5The four patterns of the pterion as described by Murphy [3]. (A) Sphenoparietal. (B) Frontotemporal. (C) Stellate. (D) Epipteric. E, epipteric bone; F, frontal bone; P, parietal bone; S, sphenoid bone; T, temporal bone. |
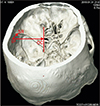 | Fig. 6Measurement of depth optic canal and the sphenoid ridge from the pterion in 3-dimensional computed tomography scan. IP-OC is the horizontal distance from the internal socation of the pterion to the lateral margin of the optic canal. IP-SR is the linear distance from the internal location of the pterion to the lateral end of the sphenoid ridge. IP, internal location of the pterion; OC, optic canal; SR, a point on the lateral end of the sphenoid ridge. |
Acknowledgements
The authors are thankful to Indian Council of Medical Research (ICMR) for funding this project.
References
1. Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MW. Gray's Anatomy: The Anatomical Basis of Medicine and Surgery. 38th ed. London: Churchill Livingstone;1995. p. 583–606.
2. Broca P. Instructions craniologiques et craniometriques. Mem Soc Anthrop Paris. 1875; 2:1–203.
3. Murphy T. The pterion in the Australian aborigine. Am J Phys Anthropol. 1956; 14:225–244.
4. Figueiredo EG, Oliveira AM, Plese JP, Teixeira MJ. Perspective of the frontolateral craniotomies. Arq Neuropsiquiatr. 2010; 68:430–432.
5. Figueiredo EG, Deshmukh P, Zabramski JM, Preul MC, Crawford NR, Spetzler RF. The pterional-transsylvian approach: an analytical study. Neurosurgery. 2006; 59:ONS263–ONS269.
6. Schick U, Dott U, Hassler W. Surgical management of meningiomas involving the optic nerve sheath. J Neurosurg. 2004; 101:951–959.
7. Ngando HM, Maslehaty H, Schreiber L, Blaeser K, Scholz M, Petridis AK. Anatomical configuration of the Sylvian fissure and its influence on outcome after pterional approach for microsurgical aneurysm clipping. Surg Neurol Int. 2013; 4:129.
8. Hyun SJ, Hong SC, Kim JS. Side selection of the pterional approach for superiorly projecting anterior communicating artery aneurysms. J Clin Neurosci. 2010; 17:592–596.
9. Ma S, Baillie LJ, Stringer MD. Reappraising the surface anatomy of the pterion and its relationship to the middle meningeal artery. Clin Anat. 2012; 25:330–339.
10. Ersoy M, Evliyaoglu C, Bozkurt MC, Konuskan B, Tekdemir I, Keskil IS. Epipteric bones in the pterion may be a surgical pitfall. Minim Invasive Neurosurg. 2003; 46:363–365.
11. Mwachaka P, Hassanali J, Odula P. Anatomic position of the pterion among Kenyans for lateral skull approaches. Int J Morphol. 2008; 26:931–933.
12. Adejuwon SA, Olopade FE, Bolaji M. Study of the location and morphology of the pterion in adult Nigerian skulls. ISRN Anat. 2013; 2013:403937.
13. Apinhasmit W, Chompoopong S, Chaisuksunt V, Thiraphatthanavong P, Phasukdee N. Anatomical consideration of pterion and its related references in Thai dry skulls for pterional surgical approach. J Med Assoc Thai. 2011; 94:205–214.
14. de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, Day L, Lam D, Jette N. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015; 17:776–783.
15. Dandy WE. The practice of surgery. The brain. Hagerstown, MD: W.F. Prior;1932. Vol. 12.
16. Cheng WY, Lee HT, Sun MH, Shen CC. A pterion keyhole approach for the treatment of anterior circulation aneurysms. Minim Invasive Neurosurg. 2006; 49:257–262.
17. Kamath V, Asif M, Bhat S, Avadhani R. A study on the pterion position variation and its neurosurgical implications. J Anat Soc India. 2016; 65:S33–S39.
18. Oguz O, Sanli SG, Bozkir MG, Soames RW. The pterion in Turkish male skulls. Surg Radiol Anat. 2004; 26:220–224.
19. Aksu F, Akyer SP, Kale A, Geylan S, Gayretli O. The localization and morphology of pterion in adult West Anatolian skulls. J Craniofac Surg. 2014; 25:1488–1491.
20. Ilknur A, Mustafa KI, Sinan B. A comparative study of variation of the pterion of human skulls from 13th and 20th century Anatolia. Int J Morphol. 2009; 27:1291–1298.
21. Urzi F, Iannello A, Torrisi A, Foti P, Mortellaro NF, Cavallaro M. Morphological variability of pterion in the human skull. Ital J Anat Embryol. 2003; 108:83–117.
22. Aydin ME, Kopuz C, Demir MT, Corumlu U, Kaya AH. Localization of pterion in neonatal cadavers: a morphometric study. Surg Radiol Anat. 2010; 32:545–550.
23. Woo EJ, Jung H, Tansatit T. Cranial index in a modern people of Thai ancestry. Anat Cell Biol. 2018; 51:25–30.
24. Ashley-Montagu MF. The anthropological significance of the pterion in the primates. Am J Phys Anthropol. 1933; 18:159–336.
25. Matsumura G, Kida K, Ichikawa R, Kodama G. Pterion and epipteric bones in Japanese adults and fetuses, with special reference to their formation and variations. Kaibogaku Zasshi. 1991; 66:462–471.
26. Hwang K, Kim JH, Baik SH. The thickness of the skull in Korean adults. J Craniofac Surg. 1999; 10:395–399.
27. Pensler J, McCarthy JG. The calvarial donor site: an anatomic study in cadavers. Plast Reconstr Surg. 1985; 75:648–651.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download