Abstract
The literature showing information regarding ovarian venous variation, its diameter and termination distance from respective renal venous origin are limited. This information is important in various surgical and clinical procedures including venous embolization, vascular reconstruction during renal transplantation and localizing the source of origin of a pelvic mass. We examined 94 sides of 47 formalin fixed female cadavers and noted the course and termination of ovarian veins. We measured the diameter of ovarian veins at their termination point and the termination distance in respect to the termination point of renal veins at inferior vena cava (IVC) on respective sides. We found two cases of variations related to right ovarian vein -one, right ovarian vein joined the right renal vein; two, right ovarian vein duplicated and joined with IVC at two different points. We found one case of variation related to left ovarian vein—a partially duplicated left ovarian vein. All the variations were unilateral. The mean diameters of right and left ovarian veins were 3.66±1.18 and 4.20±0.96 mm, respectively. The distance of termination of ovarian veins ranged from 19–40 mm and 13–41 mm, respectively from termination points of right and left renal veins at IVC on respective sides. Our study presents a set of data regarding variation of ovarian veins, diameters and termination distances which could be useful for gynecologists, surgeons and radiologists.
In present age of advanced radiological facility, laparoscopic and transplant surgeries, the knowledge about course and variation of gonadal veins is important. Anatomic and/physiologic alteration of ovarian veins may give rise to various clinical conditions including ovarian vein reflux or pelvic congestion syndrome [1]. Ovarian venous reflux is the major cause of chronic pelvic pain in women particularly of child bearing age and ovarian venous embolization or clipping could be an effective treatment of pelvic congestion syndrome [23]. Identification of ovarian veins and tracing their courses through abdomen in computed tomography (CT) is a commonly used method of localizing the ovaries in living. It helps finding out the origin of a pelvic mass whether ovarian or not [4]. During renal transplant surgeries the donor gonadal vein is often utilized for vascular reconstruction [567]. It is suspected that some variations of the gonadal venous drainage pattern could induce hemodynamic differences causing gonadal venous congestion [8]; reflux in incompetent and dilated ovarian vein may cause pelvic congestion [19].
The ovarian veins originate from a pampiniform plexus in the broad ligament around ovary and fallopian tube, then ascend upward superficial to psoas major muscle and follow a retroperitoneal course lying on posterior abdominal wall. The right ovarian vein usually drains into the inferior vena cava (IVC) whereas the left ovarian vein drains into the left renal vein in most individuals [11011].
Available literature showing evidence of variation of ovarian vein is very limited, whereas, variant drainage pattern of testicular vein has been reported relatively frequently in literature [48101213141516]. The diagnosis of testicular venous insufficiency in form of varicocele is relatively less complicated with advent of testicular color Doppler ultrasonography in addition to clinical examination of testis [17]. On the other hand, the diagnosis of pelvic congestion syndrome and left renal vein compression syndrome in women are relatively difficult to diagnose as there are no gold standard techniques-duplex ultrasound is not much helpful, catheter based intravenous pressure measurement is too expensive for a routine use, only measurement of venous diameter could be of some help [2]. Very few researchers have measured and reported the diameters of ovarian veins [918].
In present study we investigated the variations and measurements related to ovarian veins. We dissected 47 well embalmed female cadavers to find out variations in the course and termination pattern of ovarian veins. We measured the diameter of ovarian veins at their termination points, and in addition, the distance of their termination sites from nearby anatomical landmark.
This study was conducted on 94 sides of 47 formalin fixed dissection room female cadavers in the anatomy dissection lab of our institution over a period of 4 years. The mean age of the cadavers varied from 64 to 87 years.
We followed the conventional dissection techniques; the anterior abdominal wall was dissected and lifted laterally. The peritoneum and fat on posterior abdominal wall was dissected and genitourinary organs were exposed and cleaned. The ovarian veins were secured on both sides. We examined and recorded the following things: (1) The course of ovarian vessels on both sides of each cadaver, (2) The diameters of the ovarian veins at their termination, and (3) The distances between the termination sites of ovarian veins and point of termination of renal veins at IVC on respective sides (i.e., the vertical distance between the point of termination of right renal vein at IVC to the termination point of right ovarian vein at IVC on right side; and the horizontal distance between the termination point of left renal vein at IVC to the termination point of left ovarian vein at left renal vein on left side) (Fig. 1).
All the measurements were taken by calipers.
We calculated the mean and standard deviations of diameters and distances of right and left ovarian veins using Microsoft excel software (2013). We calculated the P-value using online P-value calculator (http://www.medcalc.org/calc) and correlation coefficient of two sets of ovarian venous diameters. We also calculated correlation coefficient between the set of diameters versus distances on each side.
In majority of cases, the right ovarian veins terminated at IVC and the left ovarian veins terminated at left renal vein.
Only 3 out of 94 dissected ovarian veins (0.03%) showed variation as follows.
In a 76-year-old cadaver, the right ovarian vein drained into right renal vein, it joined the right renal vein at 19 mm distal to the origin of right renal vein from IVC at a right angle. Its diameter was 5.5 mm at termination (Fig. 2). The left ovarian vein in this case normally terminated in left renal vein 38 mm distal to the termination point of left renal vein, having a termination diameter of 3 mm.
In a 64-year-old cadaver, the right ovarian vein showed duplication. Two ovarian veins were found on right side having different diameters (2 mm and 1 mm, respectively); both the ovarian veins drained into IVC—thicker one at 24 mm and thinner one at 14 mm distal to termination point of right renal vein. The thick one joined through the anterior surface of IVC while the thin one drained through the postero-lateral aspect of the IVC. Both made acute angles while joining the IVC. The left ovarian vein had a diameter of 3.6 mm and terminated at 40 mm distal to point of termination of left renal vein (Fig. 3).
In an 85-year-old cadaver, the left ovarian vein was partially duplicated. It bifurcated 40 mm distal to its termination at left renal vein. The diameters of the duplicated tributaries were 1.1 and 1.2 mm, respectively and their termination sites were separated from each other by 10 mm on left renal vein (Fig. 4). The right ovarian vein was 3.2 mm wide at its termination at IVC and the termination site was 35 mm distal to the termination point of right renal vein at IVC.
All the ovarian venous variations were unilateral. The ovarian arteries originated from abdominal aorta in all cases and no obvious variation was noted.
The average diameter of ovarian veins was 3.93±1.11 mm (range, 1–7 mm) while considering all right and left-sided ovarian veins together. The mean distances of right and left ovarian vein termination points from the points of termination of right and left renal veins at IVC were approximately 28.12±7.54 mm and 28.49±5.76 mm, respectively (Table 1).
We found the diameter of left ovarian veins were statistically significantly higher than right sided ovarian veins (P<0.05). We did not find any strong positive or negative correlations between any two groups (i.e., right vs. left diameters, right vs. left distances, diameters vs. distances on right and left sides) (Table 2).
In our dissection based study on ovarian veins, we found 0.031% variations (0.02% in course and 0.01% in termination site) of ovarian veins and all variations were unilateral. We found a set of values regarding range of diameters of right and left sided ovarian veins and their termination distances.
In a previous study by Asala et al. [13], 0.013% termination variation was noted in right sided gonadal veins which is similar to our findings; however, the previous study included both sexes. Another CT scan based study on 324 women by Koc et al. [19], reported 9.9% right ovarian vein drained into right renal vein, which was considerably different from our study and the study by Asala et al. [13], may be due to inclusion of larger population group in that study. This kind of variant drainage pattern could be responsible for pelvic congestion syndrome on right side [19]. Wong et al. [5] reported a case of duplication of right ovarian vein which terminated in IVC.
In a study by Pavkov et al. [20] on plastinated cadavers, the mean diameter of ovarian veins was 3.49 mm; in our study it was 3.93 mm. A multi-detector computed tomography based study [9] showed the mean right and left ovarian venous diameters as 2.9 and 3.2 mm, respectively. Another CT based study by Nascimento et al. [18] showed the average diameters were 4.4 mm and 4.5–6.5 mm in right and left ovarian veins, respectively. In current study, our findings (mean right and left ovarian venous diameter, 3.66 mm and 4.20 mm, respectively) fall in range with previous study-findings. We found relatively higher diameters in left ovarian veins similar to some previous studies [919] which was found to be statistically significant (P<0.05). It could be due to natural anatomical factors which make the left gonadal vein more vulnerable to congestion and dilatation [2].
Gonadal veins develop along with IVC and renal veins during early fetal life. The renal segment of IVC, the renal veins and gonadal veins are derived from development and regression of a set of fetal veins namely supracardinal veins, subcardinal veins, and the anastomoses between bilateral subcardinal and supracardinal veins. Normally, the caudal part of sub cardinal veins give rise to the gonadal veins on both sides. They drain into supra-sub cardinal anastomosis which forms part of IVC on right and part of renal vein on left side [1011]. So the usual anatomical terminations of gonadal veins are different on right and left sides.
To our knowledge, current study is the first one where the distances between the termination points of ovarian veins from the points of termination of respective renal veins were measured. The range of distance-values might help gynecologists and radiologists while performing ovarian vein embolization procedures by catheterizing respective femoral vein and while approaching ovarian vein under radiologic guidance to prevent failure of catheterization of the target vein or its inadvertent puncture [2122]. These values might help the utilization of ovarian veins for vascular reconstruction during renal transplantation surgeries too. Increase in ovarian venous diameter could be a useful parameter to diagnose and treat a case of pelvic congestion syndrome and left renal vein compression syndromes [2].
We could not find any variations related to ovarian artery in any case although previous researchers found some variations related to the course of testicular artery [23].
The major limitations of our study were- embalming procedure related limitations in cadaveric measurements and relatively lesser population size. Future studies could be planned comparing the ovarian venous diameters and distances in the living (with and without pelvic congestion syndrome) and cadavers covering a large population group to validate the current values.
In conclusion, we present here a set of data on ovarian venous diameters, termination distances from respective renal vein termination points and variations in course and termination of ovarian veins. These data are very relevant in lower abdominal and pelvic laparoscopic surgeries, vascular embolization surgeries, renal transplantation surgeries and radiological interventions and interpretations of female pelvic disorders, in order to avoid damage to this vessels or misinterpretation of radiological conditions.
Figures and Tables
 | Fig. 1The measurement points in a schematic diagram: AB, distance from right ovarian venous termination to origin of right renal vein from inferior vena cava (IVC); BC, diameter of right ovarian vein at termination; DE, distance from left ovarian venous termination to origin of left renal vein from IVC; EF, diameter of left ovarian vein at termination. AO, abdominal aorta; LK, left kidney; LOV, left ovarian vein; LRV, left renal vein; LSR, left suprarenal gland; LU, left ureter; RK, right kidney; ROV, right ovarian vein; RRV, right renal vein; RSR, right suprarenal gland; RU, right ureter. |
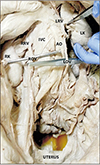 | Fig. 2Right ovarian vein draining into right renal vein. AO, abdominal aorta; IVC, inferior vena cava; LK, left kidney; LOV, left ovarian vein; LRV, left renal vein; RK, right kidney; ROV, right ovarian vein; RRV, right renal vein. |
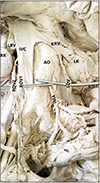 | Fig. 3Duplication of right ovarian vein. AO, abdominal aorta; IMA, inferior mesenteric artery; IVC, inferior vena cava; LK, left kidney; LOV, left ovarian vein; LRV, left renal vein; RK, right kidney; ROV, right ovarian vein; RRV, right renal vein. |
Acknowledgements
We sincerely acknowledge those kind persons, who donated their bodies for medical education and research, without whom our study would not have been possible.
References
1. Karaosmanoglu D, Karcaaltincaba M, Karcaaltincaba D, Akata D, Ozmen M. MDCT of the ovarian vein: normal anatomy and pathology. AJR Am J Roentgenol. 2009; 192:295–299.
2. Jeanneret C, Beier K, von Weymarn A, Traber J. Pelvic congestion syndrome and left renal compression syndrome: clinical features and therapeutic approaches. Vasa. 2016; 45:275–282.
3. Abdelsalam H. Clinical outcome of ovarian vein embolization in pelvic congestion syndrome. Alexandria J Med. 2016; 53:15–20.
4. Favorito LA, Costa WS, Sampaio FJ. Applied anatomic study of testicular veins in adult cadavers and in human fetuses. Int Braz J Urol. 2007; 33:176–180.
5. Wong VK, Baker R, Patel J, Menon K, Ahmad N. Renal transplantation to the ovarian vein: a case report. Am J Transplant. 2008; 8:1064–1066.
6. Veeramani M, Jain V, Ganpule A, Sabnis RB, Desai MR. Donor gonadal vein reconstruction for extension of the transected renal vessels in living renal transplantation. Indian J Urol. 2010; 26:314–316.
7. de Cerqueira JB, de Oliveira CM, Silva BG, Santos LC, Fernandes AG, Fernandes PF, Maia EL. Kidney transplantation using gonadal vein for venous anastomosis in patients with iliac vein thrombosis or stenosis: a series of cases. Transplant Proc. 2017; 49:1280–1284.
8. Gardner S. Unusual drainage of right testicular vein: a case report. Case Rep Clin Med. 2015; 4:237–240.
9. Aikimbaev K, Balli TH, Akgul E, Aksungur EH. Ovarian vein diameters measured by MDCT in women without evidence of pelvic congestion syndrome. Heart Vessels Transplant. 2017; 1:43–48.
10. Gupta R, Gupta A, Aggarwal N. Variations of gonadal veins: embryological prospective and clinical significance. J Clin Diagn Res. 2015; 9:AC08–AC10.
11. Rao S, Konduru S, Rao TR. Duplication of right ovarian vein. Arch Curr Res Int. 2017; 7:1–4.
12. Beck EM, Schlegel PN, Goldstein M. Intraoperative varicocele anatomy: a macroscopic and microscopic study. J Urol. 1992; 148:1190–1194.
13. Asala S, Chaudhary SC, Masumbuko-Kahamba N, Bidmos M. Anatomical variations in the human testicular blood vessels. Ann Anat. 2001; 183:545–549.
14. Vijisha P, Mugunthan N, Devi JR, Anbalagan J. A study of renal vein and gonadal vein variations. NJCA. 2012; 1:125–128.
15. Diwan Y, Singal R, Diwan D, Goyal S, Singal S, Kapil M. Bilateral variations of the testicular vessels: embryological background and clinical implications. J Basic Clin Reprod Sci. 2013; 2:60–62.
16. Mansilla A, Mansilla S, Pereria CJ, Russo A, Olivera E. Rare termination of the right gonadic vein. MOJ Anat Physiol. 2016; 2:177–178.
17. Belay RE, Huang GO, Shen JK, Ko EY. Diagnosis of clinical and subclinical varicocele: how has it evolved? Asian J Androl. 2016; 18:182–185.
18. Nascimento AB, Mitchell DG, Holland G. Ovarian veins: magnetic resonance imaging findings in an asymptomatic population. J Magn Reson Imaging. 2002; 15:551–556.
19. Koc Z, Ulusan S, Oguzkurt L. Right ovarian vein drainage variant: is there a relationship with pelvic varices. Eur J Radiol. 2006; 59:465–471.
20. Pavkov ML, Koebke J, Notermans HP, Brökelmann J. Quantitative evaluation of the utero-ovarian venous pattern in the adult human female cadaver with plastination? World J Surg. 2004; 28:201–205.
21. Perkov D, Vrkić Kirhmajer M, Novosel L, Popić Ramač J. Transcatheter ovarian vein embolisation without renal vein stenting for pelvic venous congestion and nutcracker anatomy. Vasa. 2016; 45:337–341.
22. Durham JD, Machan L. Pelvic congestion syndrome. Semin Intervent Radiol. 2013; 30:372–380.
23. Padur AA, Kumar N. Unique variation of the left testicular artery passing through a vascular hiatus in renal vein. Anat Cell Biol. 2019; 52:105–107.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download