Abstract
We present basic data on head positions that can serve as compensatory interventions for patients with weak tongue and buccinator muscles. We studied 30 Korean adults (15 males, 15 females; mean age, 23 years; range, 20–30 years). A TPS-100 instrument was used to measure tongue and cheek pressures and suprahyoid and buccinator muscle activities at various head rotations and tilts, as independent variables. The data were subjected to one-way analysis of variance and post-hoc (linear contrast) testing. Tongue elevation pressures differed significantly when the head was flexed or extended compared to the neutral position (P<0.01). Suprahyoid muscle activity varied significantly when the head was rotated left or right compared to neutral, or tilted with the tongue elevated (P<0.01). Cheek pressure varied significantly when the head was rotated left or right compared to neutral, or tilted (P<0.01). Both tongue and cheek pressures increased significantly when the head was extended or rotated contralaterally compared to the neutral position. Suprahyoid muscle activity increased when the head was flexed or extended, or contralaterally or ipsilaterally rotated compared to the neutral position. Therefore, we suggest that head rotation or tilting could be used to vary oral pressure and muscle activity.
Tongue muscles change in shape and position during speaking and swallowing [1]. Reductions in tongue muscle strengths trigger problems in both the pharyngeal and oral phases, particularly in terms of poor bolus formation during chewing and swallowing [2]. Tongue strength and accuracy must be improved if such problems develop; the TPS-100 device measures the maximum tongue isometric strength [34]. The buccinator muscle presses the cheek against the teeth, sending food to tooth occlusal surfaces to aid mastication [5]. Muscle strength and co-ordination may improve with training; normal functioning may resume. The strengths and activities of the tongue and buccinator muscles have been studied. However, the relationships between the muscles remain unclear.
Postural techniques improve swallowing safety by controlling food and liquid flow and reducing the aspiration risk. For example, in patients who have suffered cerebrovascular accidents, the head should be turned toward the more-involved side to close off the weaker pharyngeal wall, which makes swallowing safer [6]. In patients with pharyngeal disorders, head rotation toward the paralyzed side reduces the volume of the pyriform sinus, after which the bolus descends down the non-affected side [7], reducing pressure on the upper esophageal sphincter and pressurizing the thyroid cartilage. This in turn promotes the closure of the vocal cords [8]. Oral cancer patients who have undergone partial tongue resection can move a bolus from the mouth to the pharynx by extending the head [6]. Most stroke patients are at risk of food aspiration because the swallowing reflex is delayed. Head flexion reduces this risk by holding the bolus temporarily in the vallecula [9]. Thus, head rotations and tilts are often prescribed by occupational therapists. However, most prior studies have not explored individual swallowing physiologies. Few Korean studies have biomechanically analyzed oral pressures or suprahyoid/buccinator muscle activities.
Electromyography (EMG) is commonly used to explore movements of the upper and lower limbs. Commencing in 2017, oral pressure-measuring equipment has been increasingly used in clinics. However, no study has yet explored how head position affects oral pressure and associated muscle activities. Preliminary work with healthy adults is essential prior to studying patients with weak tongue or buccinator muscles. We thus gathered basic data on how head positioning might aid such patients, exploring how head rotation and tilt affect oral pressure and muscle activities.
The TPS-100 device (Cybermedic, Iksan, Korea) is used to analyze and strengthen tongue (front and rear) movements in patients with swallowing disorders. Tongue coordination enhances bolus movement and oral pressurization during deglutition. The device features an air bulb, a tube, a sensor, and a pressure-measuring device. We measured tongue and cheek pressures three times, and calculated averages in hPa (Fig. 1).
We used a surface EMG device (2EM 4D-MT, Relive, Gimhae, Korea) to measure suprahyoid and buccinator muscle activities. The signals were bandpass-filtered, preserving only those of 25–300 Hz. Skin resistance was minimized by removing hair and wiping the skin with an ethanol swab. The interelectrode distance was 1 cm. To measure suprahyoid activity, two electrodes were attached to the skin over the midline of the submental triangle; the ground electrode was placed on the right mastoid process. To assess right buccinator muscle activity, the first electrode was attached lateral to the mouth and the second just lateral to the first (Fig. 1) [1011]. To measure the percentage reference voluntary contraction (%RVC), each subject was asked to swallow saliva three times at intervals of 3 minutes.
We briefed recruited subjects on oral anatomical structures, our experimental plan, and the clinical significance of the study. We enrolled only volunteers. We excluded those with surgical injuries to the tongue and cheek. The subjects included 30 Korean adults (15 males, 15 females; mean age, 23 years; range, 20–30 years). The study was approved by the Dongseo University Institutional Review Board (No. 1041493-A-2019-004) and we obtained informed consent from the subjects.
Head rotation and tilt served as independent variables. The tongue pressure applied to the front of the palate when the tongue was elevated was measured, and the activities of the suprahyoid and buccinator muscles were recorded. Next, the air bulb was positioned between the right upper and lower first molars and the right buccal mucosa, and cheek pressure and suprahyoid and buccinator muscle activities were recorded during cheek contraction. All tests were run three times at 3-minute intervals. The order of the head positions tested was randomized.
The SPSS software ver. 24 (IBM Corp., Armonk, NY, USA) was used to compare all data. The level of statistical significance (α value) was set to 0.05. The following items were analyzed via one-way analysis of variance accompanied by post-hoc (linear contrast) testing: (1) oral pressures of the tongue tip and cheek in the neutral position, and after left and right head rotation; (2) oral pressures of the tongue tip and cheek in the neutral position, and after head flexion and extension; (3) buccinator and suprahyoid muscle activities in the neutral position, and after left and right head rotation; (4) buccinator and suprahyoid muscle activities in the neutral position, and after head flexion and extension.
The tongue pressure after tongue elevation did not differ when the head was in the neutral position or rotated to the left or right (F=2.95, P>0.05). When the tongue was elevated, the pressure differed significantly when the head was in the neutral position versus when it was flexed or extended (F=8.25, P<0.05). On post-hoc analysis, the difference when the head was extended compared to the neutral position remained significant (P<0.01), but the difference when the head was flexed did not (P>0.05) (Table 1, Fig. 2).
In terms of head rotation with cheek constriction, the cheek pressures differed significantly between the neutral position and upon left or right rotation (F=3.48, P<0.05). On post-hoc analysis, the difference between the neutral position and left rotation was maintained (P<0.01), but the effect of right rotation (compared to no or left rotation) was not (both P>0.05). The cheek pressure differed significantly when the head was in the neutral position versus flexed or extended (F=10.34, P<0.01). Post-hoc analysis showed that the difference upon extension (compared to neutral/flexed) (P<0.05) was maintained, but the difference upon flexion was not (both P>0.05) (Table 1, Fig. 3).
When the head was rotated with the tongue elevated, no significant difference in buccinator muscle activity between the neutral and left- or right-rotated positions was evident (F=0.33, P>0.05). In terms head flexion or extension with the tongue elevated, the buccinator muscle activity did not vary (F=0.74, P>0.05) (Table 2, Fig. 4).
No significant difference in buccinator muscle activity was noted when the head was in the neutral position versus rotated left or right (F=3.02, P>0.05). No significant difference in buccinator muscle activity was noted when the head was in the neutral position versus extended or flexed (F=1.27, P>0.05) (Table 2, Fig. 5).
In terms head rotation with the tongue elevated, the suprahyoid muscle activity varied significantly (F=255.65, P<0.01) when the head was in the neutral position versus rotated to the left or right (post-hoc analysis; both P<0.01), but the activities upon left and right rotation were equivalent (P>0.05). In terms of head flexion or extension with the tongue elevated, the suprahyoid muscle activity varied significantly (F=100.49, P<0.01) when the head was in the neutral position versus flexed or extended (post-hoc analysis; both P<0.01); the effects of flexion and extension differed (P<0.01) (Table 3, Fig. 6).
The suprahyoid muscle activity differed significantly (F=4.29, P<0.05) when the head was in the neutral position versus rotated left or right (P<0.05). The suprahyoid muscle activity differed significantly (F=4.30, P<0.05) when the head was in the neutral position versus flexed or extended (P<0.05) (Table 3, Fig. 7).
The tongue is critical in terms of food movement, and the buccinator muscles of the cheek serve as lateral retainers that prevent food particles from falling into the sulcus between the jaw and cheek [12]. Postural interventions for those exhibiting swallowing impairments have traditionally sought to functionally modify the tongue and cheek biomechanics [13]. EMG has been used to aid patients with chronic dysphagia, affording useful biofeedback on oral muscle tone, thus improving swallowing [14]. We found that the tongue and cheek pressures increased significantly when the head was extended; specifically, the cheek pressure increased on contralateral (left) head rotation. When the muscle fibers and soft tissues are stretched, the tongue and cheek pressures change; passive pressure develops when the muscle and elastic components are stretched beyond their resting lengths [15]. If a patient exhibits low tongue or cheek tension, head extension or contralateral rotation is helpful. Passive tension induced by stretching increases internal pressure, and active tensioning on contraction enhances EMG activity. Suprahyoid muscle activity increases on ipsilateral (right) rotation because the pyriform sinus volume is reduced and the bolus descends on the opposite side; the suprahyoid muscles contract [16]. These muscles elevate the hyoid either anteriorly or posteriorly. When the swallowing response is triggered, the tongue base rises to direct the bolus into the pharynx and the hyoid becomes elevated and moves anteriorly [17]. We found that the suprahyoid muscle activity was higher when the head was flexed or extended, rather than neutral. Head flexion directly moves the hyoid upward and forward because of the length-tension relationship; the force produced by the contractile elements (lying parallel to the elastic components) of the suprahyoid tendons increases [18]. Head extension widens the laryngeal vestibule and narrows the vallecular space; physiological difficulties may follow [17]. However, if oral transfer is impaired in patients who have undergone supraglottic resection, head extension is a useful postural remedy [19]. In dysphagic patients, head flexion expands the vallecular space, pushes the tongue base toward the pharynx, and protects the epiglottis. Therefore, head flexion is frequently used during deglutition training [20].
We found that the suprahyoid muscle activity increased upon contralateral (left) head rotation. Such rotation would be less favored than the neutral position in healthy adults, who use the suprahyoid muscles symmetrically. However, many stroke patients use the perioral muscles asymmetrically [21]. In left hemiplegic patients, left rotation would increase right-side muscle activity and decrease left-side muscle activity. Thus, head rotation would compensate for the suprahyoid muscle weakness of the involved side. Turning the head toward that side eliminates that region of the pharynx from muscle activation, rendering the non-impaired side more active [22].
The buccinator muscle is used to position food for chewing and to control the bolus. We found that the cheek pressure increased significantly on contralateral head rotation and extension, compensating for buccinator weakness. Cheek pressure is influenced by cheek space between fixed part (teeth, alveolar bone) and unfixed part (buccinator muscle). Head position (contralateral rotation, extension) reduced the space and pressure was increased consequently. However, there were no significant changes in the buccinator muscle activity. We assumed the origin and insertion of the muscle was hardly affected by head position [5]. The change in head position significantly increases the pressure in the cheek area even though it does not affect the buccinator muscle activity, which may be helpful for patients with buccinator weakness.
We measured oral pressure and suprahyoid and buccinator muscle activities related to head position. Both the tongue and cheek pressure increased on head extension or contralateral rotation. The suprahyoid muscle activity increased upon head flexion and extension, and contralateral and ipsilateral rotation. In conclusion, head extension or contralateral rotation would aid those with poor tongue or cheek pressure. Head flexion/extension, or contralateral/ipsilateral rotation, increase suprahyoid muscle activity; the optimal head position will vary individually in patients with functional dysphagia or who have undergone supraglottic resection.
Figures and Tables
 | Fig. 1Measurement of oral pressure and muscle activity using TPS-100 and 2EM 4D-MT devices. (A) The air bulb of TPS-100 is located in the oral cavity. The electrodes of 2EM 4D-MT are attached to the suprahyoid and buccinator muscles. (B) The TPS-100 device is consist of an air bulb, a tube, a sensor, and a pressure-measuring device. The patients provided written informed consent for the publication and the use of their images. |
 | Fig. 2Oral pressure according to head position with tongue elevation (**P<0.01). LR, left rotation; RR, right rotation; NH, neutral head; HF, head flexion; HE, head extension. |
 | Fig. 3Oral pressure according to head position with tongue elevation (*P<0.05, **P<0.01). LR, left rotation; RR, right rotation; NH, neutral head; HF, head flexion; HE, head extension. |
 | Fig. 4Muscle activity of the buccinator according to head position with tongue elevation. %RVC, percentage reference voluntary contraction; LR, left rotation; RR, right rotation; NH, neutral head; HF, head flexion; HE, head extension. |
 | Fig. 5Muscle activity of the buccinator according to head position with cheek constriction. %RVC, percentage reference voluntary contraction; LR, left rotation; RR, right rotation; NH, neutral head; HF, head flexion; HE, head extension. |
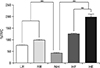 | Fig. 6Muscle activity of the suprahyoid according to head position with tongue elevation (**P<0.01). %RVC, percentage reference voluntary contraction; LR, left rotation; RR, right rotation; NH, neutral head; HF, head flexion; HE, head extension. |
 | Fig. 7Muscle activity of the suprahyoid according to head position with cheek constriction. %RVC, percentage reference voluntary contraction; LR, left rotation; RR, right rotation; NH, neutral head; HF, head flexion; HE, head extension. |
Acknowledgements
This work was supported by Dongseo University, “Dongseo Cluster Project” Research Fund of 2019 (DSU-20190002).
References
1. Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol (1985). 2014; 116:291–301.
2. Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009; 24:57–65.
3. Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008; 3:735–747.
4. Moon JH, Kim HJ, Kang MK, Won YS. Effects of tongue strength and accuracy training on tongue strength, swallowing function, and quality of life in chronic stroke patients with dysphagia. J Korea Contents Assoc. 2016; 16:605–613.
5. Drake RL, Vogl AW, Mitchell AW. Gray's anatomy for students. 4th ed. Philadelphia, PA: Elsevier/Churchill Livingstone;2019.
6. Pendleton HM, Schultz-Krohn W. Pedretti's occupational therapy: practice skills for physical dysfunction. 8th ed. St. Louis, MO.: Mosby/Elsevier;2018.
7. Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989; 70:767–771.
8. Lee H, Rho H, Cheon HJ, Oh SM, Kim YH, Chang WH. Selection of head turn side on pharyngeal dysphagia in hemiplegic stroke patients: a preliminary study. Brain Neurorehabil. 2018; 11:e19.
9. Kumai Y, Miyamoto T, Matsubara K, Samejima Y, Yoshida N, Baba H, Orita Y. Determining the efficacy of the chin-down maneuver following esophagectomy with fiberoptic endoscopic evaluation of swallowing. Arch Phys Med Rehabil. 2019; 100:1076–1084.
10. Criswell E. Cram's introduction to surface electromyography. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers;2011.
11. Taniguchi H, Tsukada T, Ootaki S, Yamada Y, Inoue M. Correspondence between food consistency and suprahyoid muscle activity, tongue pressure, and bolus transit times during the oropharyngeal phase of swallowing. J Appl Physiol (1985). 2008; 105:791–799.
12. Logemann JA, Curro FA, Pauloski B, Gensler G. Aging effects on oropharyngeal swallow and the role of dental care in oropharyngeal dysphagia. Oral Dis. 2013; 19:733–737.
13. Gonzalez-Fernandez M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: an overview. Curr Phys Med Rehabil Rep. 2013; 1:187–196.
14. Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: A retrospective evaluation. Dysphagia. 1999; 14:93–109.
15. Langenbach GE, Hannam AG. The role of passive muscle tensions in a three-dimensional dynamic model of the human jaw. Arch Oral Biol. 1999; 44:557–573.
16. Burbidge AS, Cichero JA, Engmann J, Steele CM. “A day in the life of the fluid bolus”: an introduction to fluid mechanics of the oropharyngeal phase of swallowing with particular focus on dysphagia. Appl Rheol. 2016; 26:64525.
17. Walton J, Silva P. Physiology of swallowing. Surgery. 2018; 36:529–534.
18. Okeson JP. Management of temporomandibular disorders and occlusion: e-book. 7th ed. St. Louis, MO: Elsevier Health Sciences;2014.
19. Cohen EE, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, Stubblefield MD, Abbott DM, Fisher PS, Stein KD, Lyman GH, Pratt-Chapman ML. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016; 66:203–239.
20. Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, Aydogdu I. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Arch Phys Med Rehabil. 2001; 82:1255–1260.
21. Schimmel M, Leemann B, Christou P, Kiliaridis S, Herrmann FR, Muller F. Quantitative assessment of facial muscle impairment in patients with hemispheric stroke. J Oral Rehabil. 2011; 38:800–809.
22. Logemann JA. Treatment of oral and pharyngeal dysphagia. Phys Med Rehabil Clin N Am. 2008; 19:803–816.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download