This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Controlled hypotension (CH) provides a better surgical environment and reduces operative time. However, there are some risks related to organ hypoperfusion. The EV1000/FloTrac system can provide continuous cardiac output monitoring without the insertion of pulmonary arterial catheter. The present study investigated the efficacy of this device in double jaw surgery under CH.
Methods
We retrospectively reviewed the medical records of patients who underwent double jaw surgery between 2010 and 2015. Patients were administered conventional general anesthesia with desflurane; CH was performed with remifentanil infusion and monitored with an invasive radial arterial pressure monitor or the EV1000/FloTrac system. We allocated the patients into two groups, namely an A-line group and an EV1000 group, according to the monitoring methods used, and the study variables were compared.
Results
Eighty-five patients were reviewed. The A-line group reported a higher number of failed CH (P = 0.005). A significant correlation was found between preoperative hemoglobin and intraoperative packed red blood cell transfusion (r = 0.525; P < 0.001). In the EV1000 group, the mean arterial pressure (MAP) was significantly lower 2 h after CH (P = 0.014), and the cardiac index significantly decreased 1 h after CH (P = 0.001) and 2 h after CH (P = 0.007). Moreover, venous oxygen saturation (ScVO2) decreased significantly at both 1 h (P = 0.002) and 2 h after CH (P = 0.029); however, these values were within normal limits.
Conclusion
The EV1000 group reported a lower failure rate of CH than the A-line group. However, EV1000/FloTrac monitoring did not present with any specific advantage over the conventional arterial line monitoring when CH was performed with the same protocol and same mean blood pressure. Preoperative anemia treatment will be helpful to decrease intraoperative transfusion. Furthermore, ScVO2 monitoring did not present with sufficient benefits over the risk and cost.
Go to :

Keywords: Cardiac Output, Controlled Hypotension, Osteotomy, Le Fort, Remifentanil
INTRODUCTION
Controlled hypotension (CH) or hypotensive anesthesia is one of the several anesthetic methods to decrease bleeding and blood transfusion during surgical procedures [
1]. Moreover, it provides a better surgical environment and reduces the time involved in hemostasis, thereby shortening the operative time [
2]. The current data retrieved from several articles indicate that mean arterial blood pressure (MBP) within the range of 50 to 65 mmHg is safe for young and healthy patients [
345].
Orofacial area has complex vascularization and is susceptible to excessive bleeding during orthognathic surgery [
6]. For double jaw surgery, CH has remained a common method of anesthesia owing to decreased bleeding and related complications [
1457]. Several studies on drugs and monitors used during CH have been conducted to implement CH more safely [
27891011]. However, there exists no definite standard for patient monitoring during CH. For instance, our team did not have a large experience in performing CH for more than 2 h during double jaw surgery. Moreover, we found it challenging to perform CH, and certain members of our team believed that monitoring the arterial blood pressure alone is insufficient to ensure the patient's safety. In this regard, we assumed that CH would be safe if the patient's cardiac output stayed within the normal range although the blood pressure was lower than that from the pre-anesthetic status. However, cardiac output monitoring requires invasive procedures such as pulmonary artery catheter insertion and is susceptible to risks of complications and increased medical costs.
The EV1000/ FloTrac system (Edwards Lifesciences; Irvine, CA, USA) can provide valuable data on continuous cardiac output, cardiac index, and pulse pressure variation, and requires minimally invasive procedures when compared to pulmonary arterial catheterization [
12]. Furthermore, the venous oxygen saturation of the superior vena cava could be monitored using the PreSep Oximetry Catheter connected with the EV1000 system [
13]. We tried to use these devices in patients who were undergoing double jaw surgery to improve their safety while performing CH. However, there were differences of opinion within our team over the usefulness of the equipment and its cost-effectiveness. For several years, there was no definite guideline for the monitoring devices for CH during double jaw surgery, and anesthesiologists selected the device according to their preference. The present study assessed the effectiveness of these monitoring devices over the use of continuous radial arterial blood pressure monitoring alone.
Go to :

MATERIALS AND METHODS
The study protocol was approved by the Institutional Review Board of Dankook University Hospital (DKUH IRB No. 2019-02-008). We retrospectively reviewed the anesthetic and hospital records of patients who were treated at the Department of Oromaxillofacial Surgery of the Dankook University Hospital (Cheonan, Republic of Korea) between 2010 and 2015. Patients were identified using the hospital database of medical records, which were searched using the following keywords for operation name: “Le Fort I osteotomy,” “double jaw,” and “bilateral sagittal split ramus osteotomy (BSSRO).”
Inclusion criteria included patients with age ranging within 18 to 40 years who underwent double jaw surgery (Le Fort I osteotomy with BSSRO), more than 2 h of continuous CH (target mean arterial pressure [MAP]: 55–65 mmHg) with remifentanil and desflurane anesthesia, and arterial blood gas analysis every hour. Exclusion criteria included the following: Patients under the age of 18 years and those above 40 years; patients with a history of systemic diseases (hypertension and diabetes mellitus), short operative time (less than 2.5 h), different methods of performing CH, inadequate documentation of anesthetic records, inadequate laboratory data during anesthesia, and history of congenital abnormality (e.g., cleft palate). Patients were assigned to two groups as follows: those who were monitored using only the continuous radial artery pressure monitoring device were assigned to the A-line group and those monitored using the EV1000/FloTrac device were assigned to the EV1000 group.
Selected patients were anesthetized using the same protocol and the same surgeon performed the surgery. Patients were monitored with conventional monitoring devices including automated cuff for measuring the blood pressure, arterial oxygen saturation, and electrocardiogram. Anesthetic induction was performed with propofol 1.5 mg/kg, rocuronium 0.6 mg/kg, and remifentanil infusion. Anesthesia was maintained with 7 to 10 vol% of desflurane and intermittent dose of rocuronium. CH was achieved with increasing remifentanil infusion of 0.05 to 0.5 µg/kg/min and the target MBP ranged from 55 to 65 mmHg. We did not use any other hypotensive drug other than remifentanil. We used remifentanil instead of vasodilators to perform CH because we assumed that proper pain control with remifentanil would decrease the hypertensive reaction to painful surgical procedures that facilitated CH. Invasive pressure monitoring with radial arterial catheter insertion was performed in both the groups. The A-line group was monitored with the invasive radial arterial pressure monitor only, whereas the EV1000/FloTrac system and continuous venous oxygen saturation monitoring central venous catheter (Edwards Lifesciences) were used to monitor the cardiac output and venous oxygen saturation in the EV1000 group. In the A-line group, fluid therapy was performed with a crystalloid solution with or without a colloid solution. If the heart rate was higher than 90/minute, volume challenge with 300 mL of crystalloid solution was performed. If the total crystalloid solution infusion was more than 2000 mL, the volume change was switched to a colloid solution. The colloid solution was infused to replace the blood loss. The target urine output was 1 mL/kg, and PRBC transfusion was performed if the hemoglobin level was lower than 9 g/dL during the surgery. Intravenous ephedrine 5 mg was used when the systolic pressure lowered to below 70 mmHg, and the remifentanil dose was adjusted.
In the EV1000 group, goal-directed volume management was performed, target stroke volume variation was under 10%, and if it increased over 10%, volume challenge was performed with 300 mL of crystalloid solution. The colloid infusion protocol was the same as that in the A-line group. The target urine output was 1 mL/kg and the PRBC transfusion was performed if the hemoglobin level was lower than 9 g/dL during the surgery. The target cardiac index was over 2.5 L/min/m2, and if it decreased below 2.5 L/min/m2, we administered an intravenous injection of ephedrine 5 mg. The target ScVO2 was maintained over 75%.
Data on changes in the arterial blood gas analysis during CH, the volume of fluid infusion, operative time, intensive care unit stay time, hospital stay, and perioperative blood transfusion during double jaw surgery were collected. Data on blood pressure, blood gas analysis, cardiac index, ScVO2, and urine output were collected at pre-CH, 1 h after the induction of CH (CH 1 h), 2 h after CH (CH 2 h), and 1 h after the end of CH.
The total fluid volume during the surgery was recorded as crystalloid, colloidal, and transfused red blood cells per body weight. Comparisons between the two groups were performed using the paired t-test, and patients' demographic data were analyzed using the Chi-square and Fisher's exact tests. Linear regression tests were performed to analyze the variables associated with the transfusion of packed red blood cells. Statistical analyses were performed using the SPSS 18.0 (IBM Corporation; NY, USA). P-values less than 0.05 were considered statistically significant.
Go to :

RESULTS
Based on the data collected from the hospital database, 85 patients who had undergone double jaw surgery (Le Fort I osteotomy with bilateral sagittal split osteotomy) were included in the study. Thirty-five patients were allocated to the EV1000 group and were monitored using the EV1000 system, whereas 50 patients were allocated to the A-line group. We excluded 24 patients owing to incomplete records and inappropriate CH (
Fig. 1). Patients in the A-line group reported a greater number of failed CH (P = 0.005). Finally, 61 patients reported successful CH. Out of these 61 patients, 31 were included in the EV1000 group and 30 were included in the A-line group.
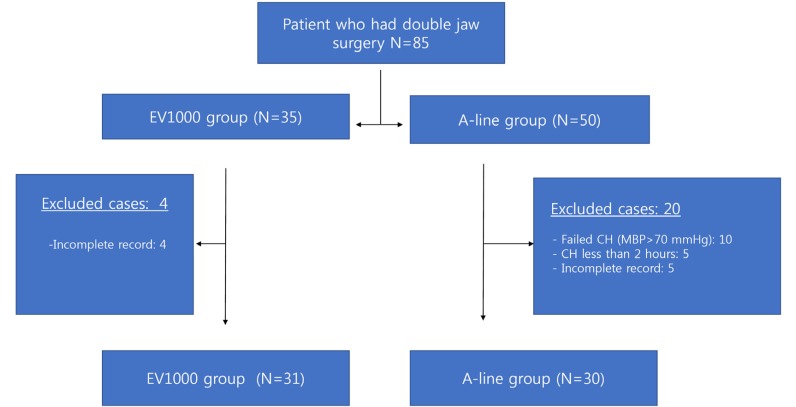 | Fig. 1Flow chart of the included cases. CH: controlled hypotension, N: number of patients, MBP: mean arterial blood pressure
|
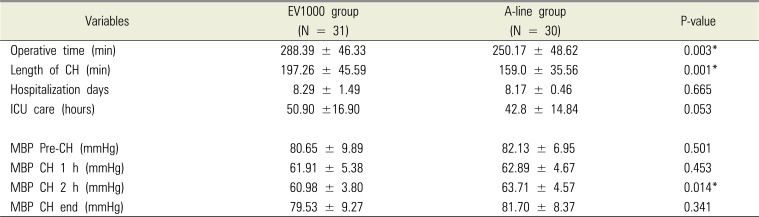
There were no statistical differences between the two groups in terms of age, sex, height, body weight, and the class of ASA (
Tables 1 and
2). The operative time and length of CH in the A-line group were significantly shorter than those in the EV1000 group (operative time: P = 0.003, length of CH: P = 0.001;
Table 2). There was no statistical difference between the two groups in the days of hospitalization and intensive care unit admission time (
Table 2).
Table 1
Patient characteristics (there were no differences between the two groups)

|
Patient characteristics |
EV1000 group |
A-line group |
P-value |
|
Age (years) |
23.55 ± 4.56 |
22.03 ± 4.78 |
0.211 |
|
Body weight (kg) |
65.62 ± 14.61 |
64.00 ± 11.31 |
0.632 |
|
Height (cm) |
169.48 ± 8.39 |
168.63 ± 7.66 |
0.681 |
|
Sex (male / female) |
12 / 19 |
12 / 18 |
0.563 |
|
ASA (Class I / II) |
30/ 1 |
30 / 0 |
0.508 |

Table 2
Time-related results and mean arterial blood pressure during the operation
|
Variables |
EV1000 group (N = 31) |
A-line group (N = 30) |
P-value |
|
Operative time (min) |
288.39 ± 46.33 |
250.17 ± 48.62 |
0.003*
|
|
Length of CH (min) |
197.26 ± 45.59 |
159.0 ± 35.56 |
0.001*
|
|
Hospitalization days |
8.29 ± 1.49 |
8.17 ± 0.46 |
0.665 |
|
ICU care (hours) |
50.90 ±16.90 |
42.8 ± 14.84 |
0.053 |
|
MBP Pre-CH (mmHg) |
80.65 ± 9.89 |
82.13 ± 6.95 |
0.501 |
|
MBP CH 1 h (mmHg) |
61.91 ± 5.38 |
62.89 ± 4.67 |
0.453 |
|
MBP CH 2 h (mmHg) |
60.98 ± 3.80 |
63.71 ± 4.57 |
0.014*
|
|
MBP CH end (mmHg) |
79.53 ± 9.27 |
81.70 ± 8.37 |
0.341 |

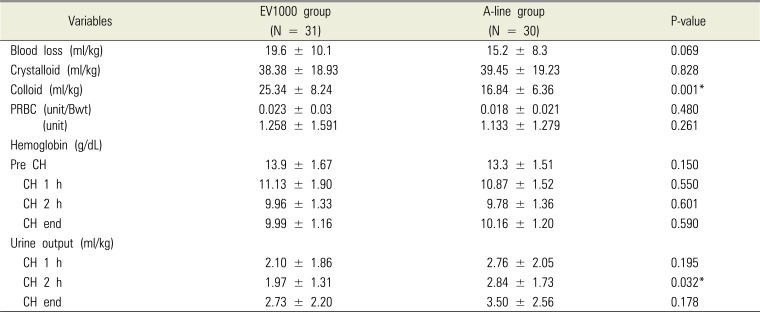
The MBP during surgery was not significantly different, except for the CH 2 h reported in the EV1000 group that showed significantly lower MBP (P = 0.029). There was no difference between the two groups with respect to the crystalloid solution infusion volume and transfusion. However, the colloid infusion volume was significantly larger in the EV1000 group (P = 0.001,
Table 3). There was no difference between the two groups with regard to the results of changes in the intraoperative blood gas analysis. The EV1000 group showed slightly less volume of urine output in CH 2 h. However, both the groups reported more than 1 mL/kg/h of urine output (
Table 3).
Table 3
Estimated blood loss, urine output, and infused fluids (crystalloid, colloid, and PRBC)
|
Variables |
EV1000 group (N = 31) |
A-line group (N = 30) |
P-value |
|
Blood loss (ml/kg) |
19.6 ± 10.1 |
15.2 ± 8.3 |
0.069 |
|
Crystalloid (ml/kg) |
38.38 ± 18.93 |
39.45 ± 19.23 |
0.828 |
|
Colloid (ml/kg) |
25.34 ± 8.24 |
16.84 ± 6.36 |
0.001*
|
|
PRBC (unit/Bwt) |
0.023 ± 0.03 |
0.018 ± 0.021 |
0.480 |
|
(unit) |
1.258 ± 1.591 |
1.133 ± 1.279 |
0.261 |
|
Hemoglobin (g/dL) |
|
|
|
|
Pre CH |
13.9 ± 1.67 |
13.3 ± 1.51 |
0.150 |
|
CH 1 h |
11.13 ± 1.90 |
10.87 ± 1.52 |
0.550 |
|
CH 2 h |
9.96 ± 1.33 |
9.78 ± 1.36 |
0.601 |
|
CH end |
9.99 ± 1.16 |
10.16 ± 1.20 |
0.590 |
|
Urine output (ml/kg) |
|
|
|
|
CH 1 h |
2.10 ± 1.86 |
2.76 ± 2.05 |
0.195 |
|
CH 2 h |
1.97 ± 1.31 |
2.84 ± 1.73 |
0.032*
|
|
CH end |
2.73 ± 2.20 |
3.50 ± 2.56 |
0.178 |

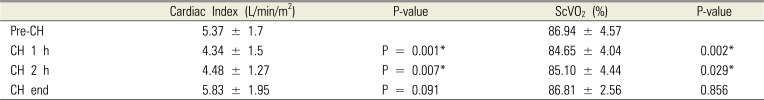
In the EV1000 group, the cardiac index significantly decreased at CH 1 h (P = 0.001) and CH 2 h (P = 0.007). Moreover, the ScVO
2 decreased significantly at CH 1 h (P = 0.002) and CH 2 h (P = 0.029). However, these were within normal limits (
Table 4). There was a significant correlation between the preoperative hemoglobin and intraoperative PRBC transfusion values (r = 0.525; P < 0.001;
Fig. 2); however, the values were otherwise unremarkable (
Table 5). One patient in the A-line group had a wound infection in the oral cavity on the third postoperative day that was surgically treated; the patient recovered without any other complications. There was no specific complication related to CH after the surgery.
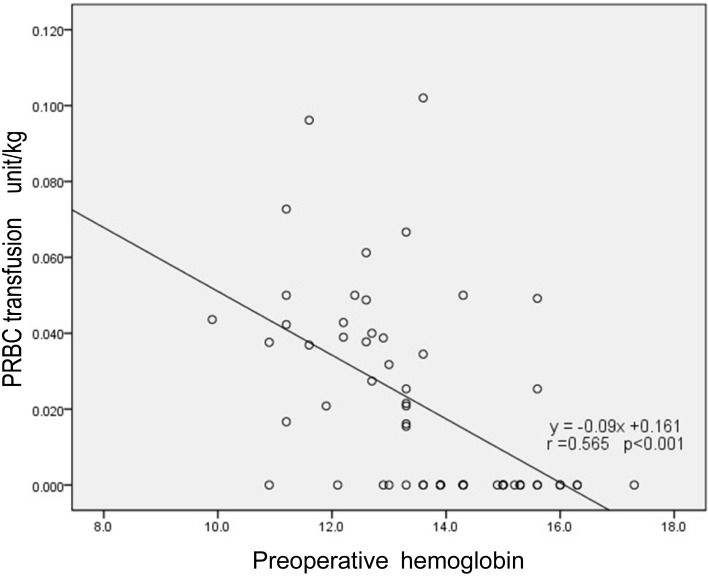 | Fig. 2Scatter plot with regression line between preoperative hemoglobin and packed red blood cells transfusion.
|
Table 4
Changes of Cardiac index and ScVO2 from Pre-CH to CH 1 h, CH 2 h, and CH end during controlled hypotension in EV1000 group
|
Cardiac Index (L/min/m2) |
P-value |
ScVO2 (%) |
P-value |
|
Pre-CH |
5.37 ± 1.7 |
|
86.94 ± 4.57 |
|
|
CH 1 h |
4.34 ± 1.5 |
P = 0.001*
|
84.65 ± 4.04 |
0.002*
|
|
CH 2 h |
4.48 ± 1.27 |
P = 0.007*
|
85.10 ± 4.44 |
0.029*
|
|
CH end |
5.83 ± 1.95 |
P = 0.091 |
86.81 ± 2.56 |
0.856 |

Table 5
Associations of intraoperative transfusion of PRBC with preoperative hemoglobin, operation time, controlled hypotension time, and mean arterial pressure by univariate linear regression analyses
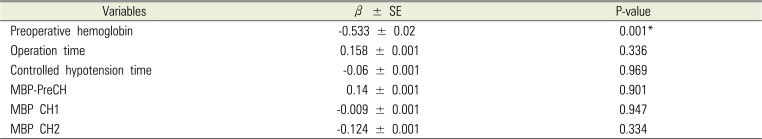
|
Variables |
β ± SE |
P-value |
|
Preoperative hemoglobin |
-0.533 ± 0.02 |
0.001*
|
|
Operation time |
0.158 ± 0.001 |
0.336 |
|
Controlled hypotension time |
-0.06 ± 0.001 |
0.969 |
|
MBP-PreCH |
0.14 ± 0.001 |
0.901 |
|
MBP CH1 |
-0.009 ± 0.001 |
0.947 |
|
MBP CH2 |
-0.124 ± 0.001 |
0.334 |

Go to :

DISCUSSION
The present study did not report any significant differences in the perioperative MAP (except at CH 2 h), variables of arterial blood gas analysis, crystalloid solution infusion, blood loss, transfusion, hospitalization days, and ICU admission. Patients in the EV1000 group reported significantly less MBP and urine output 2 h after CH; however, it was higher than the target urine output (1 mL/kg/h). Both groups were treated with a similar method of CH, except for the monitoring device, and both groups successfully achieved acceptable CH that followed a similar outcome. However, 10 patients of the A-line group were excluded (none in the EV1000 group) owing to failure to perform acceptable CH; the EV1000 group showed significantly lower MBP at CH 2 h. A possibility could be that the information from EV1000 devices showed that adequate blood flow was maintained when performing CH, allowing more aggressive treatment. The pulse pressure variation was a good parameter for proper fluid management that resulted in more volume replacement [
14].
We observed that the cardiac index and ScVO
2 decreased significantly; however, these values were within normal limits and arterial blood gas results remained normal while performing CH. These findings confirmed the safety of CH from 50 to 65 mmHg in young healthy patients that was reported previously [
345]. In this regard, the EV1000/FloTrac device might be helpful to successfully maintain CH.
Although monitoring devices provide useful information, the equipment are expensive and the application is invasive with related complications. We need to consider these aspects while selecting the monitoring devices for CH. Based on the results of the present study, we would like to consider the use of the EV1000/FloTrac device to monitor continuous cardiac output. However, ScVO
2 monitoring did not provide enough benefits for 2 to 3 h of CH in young healthy patients. It significantly decreased during CH; however, the values were always more than 80% at all time points and the target ScVO
2 value in a critically ill patient was maintained over 70%. Moreover, all patients reported better scores [
15]. Furthermore, we required subclavian central venous catheter insertion to monitor ScVO
2 that has its own risks of several complications including infection, subclavian artery puncture, hemothorax, and pneumothorax [
16].
The higher preoperative hemoglobin found in patients correlated with low intraoperative PRBC transfusion values (
Fig. 2). If a patient is anticipating double jaw surgery and has low preoperative hemoglobin, aggressive preoperative anemia management should be considered to decrease the perioperative transfusion [
17].
The present study has several limitations. This is a retrospective review of the cases from the hospital medical database. Although we used the same anesthetic protocol including the same drugs, name for the procedure, monitoring device, and target blood pressure, this study was neither prospective nor randomized. The timing of the patient's operation was not evenly distributed. We collected data from 2010 to 2015, and all patients operated on from 2015 were allocated to the A-line group and none to the EV1000 group owing to the anesthesiologist's preference. After 2015, the surgeon's skill had improved, resulting in reduced CH time (less than 2 h). Moreover, we could not include more patients after 2015. This probably was the reason for the shorter operative time in the A-line group than in the EV1000 group.
In conclusion, the use of EV1000/FloTrac monitoring system showed lesser CH failures; however, there was no presenting specific advantage over the conventional arterial line monitoring device if CH was performed with the same protocol and the same MBP. The EV1000/FloTrac device provided information related to systemic circulation, including continuous cardiac output and pulse pressure variation, and proved advantageous over the conventional arterial line transducer when performing CH. The MBP ranging from 55 to 65 mmHg was considered safe with regard to results including arterial blood gas analysis, urine output, and ScVO2. The ScVO2 monitoring did not present enough benefits over its risks and costs involved in a well-managed CH (MBP 55–65 mmHg) in young healthy patients.
Go to :

ACKNOWLEDGEMENTS
This study was financially supported by Dankook University.
Go to :

Notes
Go to :

References
1. Varol A, Basa S, Ozturk S. The role of controlled hypotension upon transfusion requirement during maxillary downfracture in double-jaw surgery. J Craniomaxillofac Surg. 2010; 38:345–349. PMID:
19913434.

2. Jangra K, Malhotra SK, Gupta A, Arora S. Comparison of quality of the surgical field after controlled hypotension using esmolol and magnesium sulfate during endoscopic sinus surgery. J Anaesthesiol Clin Pharmacol. 2016; 32:325–328. PMID:
27625479.

3. Dolman RM, Bentley KC, Head TW, English M. The effect of hypotensive anesthesia on blood loss and operative time during Le Fort I osteotomies. J Oral Maxillofac Surg. 2000; 58:834–839. discussion 40. PMID:
10935580.

4. Ettinger KS, Yildirim Y, Weingarten TN, Van Ess JM, Viozzi CF, Arce K. Hypotensive Anesthesia Is Associated With Shortened Length of Hospital Stay Following Orthognathic Surgery. J Oral Maxillofac Surg. 2016; 74:130–138. PMID:
26047710.

5. Carlos E, Monnazzi MS, Castiglia YM, Gabrielli MF, Passeri LA, Guimaraes NC. Orthognathic surgery with or without induced hypotension. Int J Oral Maxillofac Surg. 2014; 43:577–580. PMID:
24331734.

6. Park SY, Seo KS, Karm MH. Perioperative red blood cell transfusion in orofacial surgery. J Dent Anesth Pain Med. 2017; 17:163–181. PMID:
29090247.

7. Ervens J, Marks C, Hechler M, Plath T, Hansen D, Hoffmeister B. Effect of induced hypotensive anaesthesia vs isovolaemic haemodilution on blood loss and transfusion requirements in orthognathic surgery: a prospective, single-blinded, randomized, controlled clinical study. Int J Oral Maxillofac Surg. 2010; 39:1168–1174. PMID:
20961738.

8. Gillespie R, Shishani Y, Streit J, Wanner JP, McCrum C, Syed T. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012; 94:1284–1290. PMID:
22810398.

9. Shear T, Tobias JD. Cerebral oxygenation monitoring using near infrared spectroscopy during controlled hypotension. Paediatr Anaesth. 2005; 15:504–508. PMID:
15910352.

10. Yazigi A, Madi-Jebara S, Haddad F, Hayek G, Jawish D. Accuracy of radial arterial pressure measurement during surgery under controlled hypotension. Acta Anaesthesiol Scand. 2002; 46:173–175. PMID:
11942865.

11. Tobias JD. Nicardipine for controlled hypotension during orthognathic surgery. Plast Reconstr Surg. 1997; 99:1539–1543. PMID:
9145121.

12. Maeda T, Hamaguchi E, Kubo N, Shimokawa A, Kanazawa H, Ohnishi Y. The accuracy and trending ability of cardiac index measured by the fourth-generation FloTrac/Vigileo system and the Fick method in cardiac surgery patients. J Clin Monit Comput. 2019; 33:767–776. PMID:
30406422.

13. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. 2010; 55:40–46.e1. PMID:
19854541.

14. Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul JL. The changes in pulse pressure variation or stroke volume variation after a “tidal volume challenge” reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med. 2017; 45:415–421. PMID:
27922879.

15. Hartog C, Bloos F. Venous oxygen saturation. Best Pract Res Clin Anaesthesiol. 2014; 28:419–428. PMID:
25480771.

16. Shin HJ, Na HS, Koh WU, Ro YJ, Lee JM, Choi YJ. Complications in internal jugular vs subclavian ultrasound-guided central venous catheterization: a comparative randomized trial. Intensive Care Med. 2019; 45:968–976. PMID:
31143996.

17. Koo BN, Kwon MA, Kim SH, Kim JY, Moon YJ, Park SY. Korean clinical practice guideline for perioperative red blood cell transfusion from Korean Society of Anesthesiologists. Korean J Anesthesiol. 2019; 72:91–118. PMID:
30513567.
Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download