Abstract
Background
Methods
Results
Conclusions
Figures and Tables
 | Fig. 1MTT assays showed that the viability of amniotic epithelial cells was not affected by different concentrations of remifentanil |
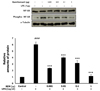 | Fig. 3Preconditioning with remifentanil reduced NF-κB expression after inducing inflammation in cells using with lipopolysaccharide (LPS) in the cells.
##P < 0.01, ###P < 0.001 versus control group; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS group.
|
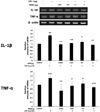 | Fig. 4RT-PCR analysis showed that preconditioning with remifentanil reduced IL-1β and TNF-α expression after inducing inflammation in cells using lipopolysaccharide (LPS).
##P < 0.01, ###P < 0.001 versus control group; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS group.
|
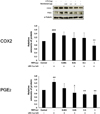 | Fig. 5Preconditioning with remifentanil reduced PGE2 expression after inducing inflammation in cells using lipopolysaccharide (LPS). The expression of COX2 tended to decrease non-significantly at low concentrations and was statistically significant at the highest concentration of remifentanil (1 µg).
##P < 0.01, ###P < 0.001 versus control group; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS group.
|
ACKNOWLEDGMENT
Notes
AUTHOR CONTRIBUTIONS Cheul-Hong Kim: Conceptualization.
Sang-Hoon Lee: Data curation, Formal analysis.
Eun-Jung Kim: Formal analysis, Investigation, Methodology.
Ji-Hye Ahn: Investigation, Methodology.
Eun-Ji Choi: Data curation, Formal analysis, Methodology.
Ji-Uk Yoon: Supervision, Writing – review & editing.
In-Seok Choi: Conceptualization, Writing – original draft, Writing – review & editing.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download