INTRODUCTION
1. An overview of early childhood caries
Table 1
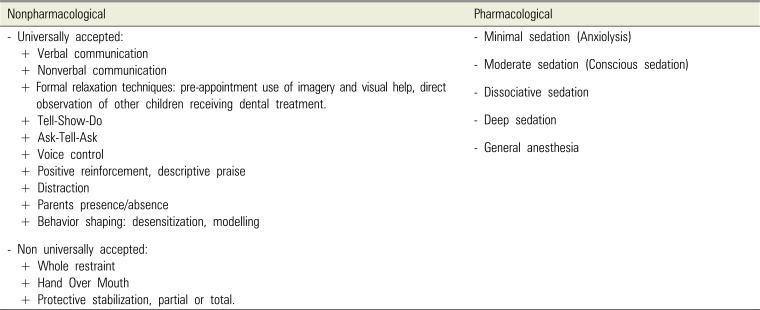
Classification of behavioral management techniques in pediatric dentistry
2. General anesthesia in the management of ECC
2.1 Definition and indications of GA
• The degree of non-cooperation of the child due to a lack of psychological or emotional maturity and/or mental, physical, or medical disability.
• The degree of anxiety exhibited, which can lead to an uncommunicative child.
• The presence of complex medical conditions.
• The very young age of the patient, which might contra-indicate the use of other sedation strategies in the anesthesia continuum.
• The failure of other behavioral guidance modalities.
• The duration of the intervention and need for immediate pain relief or significant surgical procedures.
• The presence of allergies that absolutely contraindicate local anesthesia, the presence of an acute infection, or anatomic variations that render local anesthesia ineffective.
2.2 Mortality and morbidity risks of pediatric GA
2.3 The increasing use of GA in pediatric dentistry
Table 2
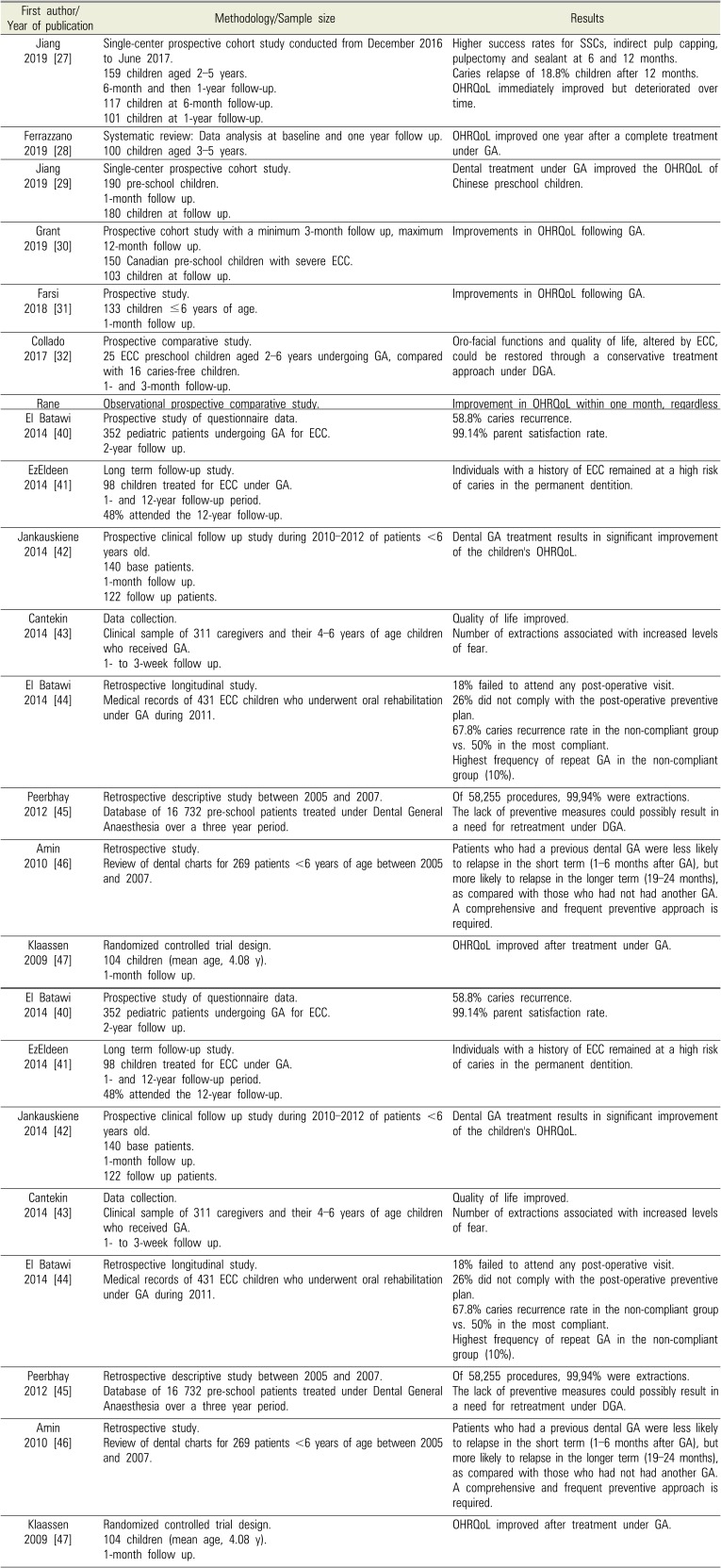
ECC dental rehabilitation under GA around the world
| First author/Year of publication | Methodology/Sample size | Results |
|---|---|---|
| Jiang 2019 [27] | Single-center prospective cohort study conducted from December 2016 to June 2017. | Higher success rates for SSCs, indirect pulp capping, pulpectomy and sealant at 6 and 12 months. |
| 159 children aged 2–5 years. | Caries relapse of 18.8% children after 12 months. | |
| 6-month and then 1-year follow-up. | OHRQoL immediately improved but deteriorated over time. | |
| 117 children at 6-month follow-up. | ||
| 101 children at 1-year follow-up. | ||
| Ferrazzano 2019 [28] | Systematic review: Data analysis at baseline and one year follow up. | OHRQoL improved one year after a complete treatment under GA. |
| 100 children aged 3–5 years. | ||
| Jiang 2019 [29] | Single-center prospective cohort study. | Dental treatment under GA improved the OHRQoL of Chinese preschool children. |
| 190 pre-school children. | ||
| 1-month follow up. | ||
| 180 children at follow up. | ||
| Grant 2019 [30] | Prospective cohort study with a minimum 3-month follow up, maximum 12-month follow up. | Improvements in OHRQoL following GA. |
| 150 Canadian pre-school children with severe ECC. | ||
| 103 children at follow up. | ||
| Farsi 2018 [31] | Prospective study. | Improvements in OHRQoL following GA. |
| 133 children ≤6 years of age. | ||
| 1-month follow up. | ||
| Collado 2017 [32] | Prospective comparative study. | Oro-facial functions and quality of life, altered by ECC, could be restored through a conservative treatment approach under DGA. |
| 25 ECC preschool children aged 2–6 years undergoing GA, compared with 16 caries-free children. | ||
| 1- and 3-month follow-up. | ||
| Rane 2017 [33] | Observational prospective comparative study. | Improvement in OHRQoL within one month, regardless of GA or LA. |
| 50 parents of children aged 2–6 years old with ECC divided into 2 groups: 25 parents of children treated under GA, 25 parents of children treated under LA. | ||
| 1-month follow up. | ||
| Chao 2017 [34] | Retrospective study of data collection. | Children OHRQoL improved significantly. |
| 659 pediatric patients treated for ECC under GA from 2013–2014. One month follow up. | 82.8% of families reported a high degree of satisfaction. | |
| Amin 2016 [35] | Retrospective cohort study. | Amalgam restorations and SSCs showed longer survival rates than composite restorations. |
| 818 ECC children, ≤72 months at the time of treatment. | Higher survival rate of pulpectomies compared to indirect pulp capping and pulpotomies. | |
| 3-year follow up. | 32.9% required retreatment over the 3 year follow up. | |
| Wong 2016 [36] | Data collection. | Emergency dental extraction under GA significantly improved the OHRQoL of preschool children who presented to the emergency department with the consequences of untreated dental caries. |
| 221 preschool children who underwent emergency extractions under GA over a 12-month period. | ||
| 2-week follow up. | ||
| 126 children at follow up. | ||
| De Souza 2016 [37] | Cohort study. | Substantial improvements in parents' ratings of their children's OHRQoL. |
| 126 children at follow up. | ||
| 78 parents of 2–6 year old children with ECC undergoing GA. | ||
| A minimum of 1-month follow-up. | ||
| 72 parents follow-up. | ||
| Yawary 2016 [38] | Data collection, parents' questionnaire. | OHRQoL of children less than 6 years of age was improved after comprehensive oral rehabilitation under GA, improvement sustained over a three month period. |
| 70 parents of preschool children under age 6 undergoing oral rehabilitation under GA. | ||
| Two weeks and then 3-month follow-up. | ||
| 39 parents at follow up. | ||
| Amin 2015 [39] | Single center retrospective cohort study. | Caries relapse rate of 21.6%. |
| 278 children <6 years of age at the time of GA. | ASA-2 children and those with less than full primary dentition during GA were twice as likely to experience caries relapse. | |
| 36-month follow up period over 5 recall visits. | ||
| 45.3% children returned for all recall visits. | ||
| El Batawi 2014 [40] | Prospective study of questionnaire data. | 58.8% caries recurrence. |
| 352 pediatric patients undergoing GA for ECC. | 99.14% parent satisfaction rate. | |
| 2-year follow up. | ||
| EzEldeen 2014 [41] | Long term follow-up study. | Individuals with a history of ECC remained at a high risk of caries in the permanent dentition. |
| 98 children treated for ECC under GA. | ||
| 1- and 12-year follow-up period. | ||
| 48% attended the 12-year follow-up. | ||
| Jankauskiene 2014 [42] | Prospective clinical follow up study during 2010–2012 of patients <6 years old. | Dental GA treatment results in significant improvement of the children's OHRQoL. |
| 140 base patients. | ||
| 1-month follow up. | ||
| 122 follow up patients. | ||
| Cantekin 2014 [43] | Data collection. | Quality of life improved. |
| Clinical sample of 311 caregivers and their 4–6 years of age children who received GA. | Number of extractions associated with increased levels of fear. | |
| 1- to 3-week follow up. | ||
| El Batawi 2014 [44] | Retrospective longitudinal study. | 18% failed to attend any post-operative visit. |
| Medical records of 431 ECC children who underwent oral rehabilitation under GA during 2011. | 26% did not comply with the post-operative preventive plan. | |
| 67.8% caries recurrence rate in the non-compliant group vs. 50% in the most compliant. | ||
| Highest frequency of repeat GA in the non-compliant group (10%). | ||
| Peerbhay 2012 [45] | Retrospective descriptive study between 2005 and 2007. | Of 58,255 procedures, 99,94% were extractions. |
| Database of 16 732 pre-school patients treated under Dental General Anaesthesia over a three year period. | The lack of preventive measures could possibly result in a need for retreatment under DGA. | |
| Amin 2010 [46] | Retrospective study. | Patients who had a previous dental GA were less likely to relapse in the short term (1–6 months after GA), but more likely to relapse in the longer term (19–24 months), as compared with those who had not had another GA. A comprehensive and frequent preventive approach is required. |
| Review of dental charts for 269 patients <6 years of age between 2005 and 2007. | ||
| Klaassen 2009 [47] | Randomized controlled trial design. | OHRQoL improved after treatment under GA. |
| 104 children (mean age, 4.08 y). | ||
| 1-month follow up. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download