1. Whitmore WF Jr. Hormone therapy in prostatic cancer. Am J Med. 1956; 21:697–713. PMID:
13362301.

2. Beahrs OH, Henson DE, Hutter RVP, Kennedy BJ. American Joint Committee on Cancer. Manual for staging of cancer. 4th ed. Philadelphia: J.B. Lippincott Company;1992.
3. Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, et al. American Joint Committee on Cancer. AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven;1997.
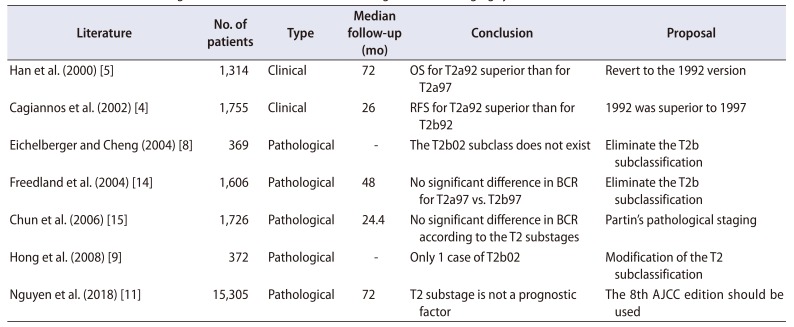
4. Cagiannos I, Graefen M, Karakiewicz PI, Ohori M, Eastham JA, Rabbani F, et al. Analysis of clinical stage T2 prostate cancer: do current subclassifications represent an improvement? J Clin Oncol. 2002; 20:2025–2030. PMID:
11956261.

5. Han M, Walsh PC, Partin AW, Rodriguez R. Ability of the 1992 and 1997 American Joint Committee on Cancer staging systems for prostate cancer to predict progression-free survival after radical prostatectomy for stage T2 disease. J Urol. 2000; 164:89–92. PMID:
10840430.

6. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. American Joint Committee on Cancer. AJCC cancer staging manual. 6th ed. New York: Springer;2002.
7. van der Kwast TH, Amin MB, Billis A, Epstein JI, Griffiths D, Humphrey PA, et al. ISUP Prostate Cancer Group. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol. 2011; 24:16–25. PMID:
20818340.

8. Eichelberger LE, Cheng L. Does pT2b prostate carcinoma exist? Critical appraisal of the 2002 TNM classification of prostate carcinoma. Cancer. 2004; 100:2573–2576. PMID:
15197798.

9. Hong SK, Han BK, Chung JS, Park DS, Jeong SJ, Byun SS, et al. Evaluation of pT2 subdivisions in the TNM staging system for prostate cancer. BJU Int. 2008; 102:1092–1096. PMID:
18671786.

10. Antunes HP, Parada B, Carvalho J, Eliseu M, Jarimba R, Oliveira R, et al. Prognostic value of subclassification (pT2 stage) of pathologically organ-confined prostate cancer: confirmation of the changes introduced in the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. Arch Ital Urol Androl. 2018; 90:191–194. PMID:
30362685.

11. Nguyen DP, Vertosick EA, Sharma V, Corradi RB, Vilaseca A, Takeda T, et al. Does subclassification of pathologically organ confined (pT2) prostate cancer provide prognostic discrimination of outcomes after radical prostatectomy? J Urol. 2018; 199:1502–1509. PMID:
29307681.

12. Paner GP, Stadler WM, Hansel DE, Montironi R, Lin DW, Amin MB. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018; 73:560–569. PMID:
29325693.

13. Fine SW. Evolution in prostate cancer staging: pathology updates from AJCC 8th edition and opportunities that remain. Adv Anat Pathol. 2018; 25:327–332. PMID:
29870405.

14. Freedland SJ, Partin AW, Epstein JI, Walsh PC. Biochemical failure after radical prostatectomy in men with pathologic organ-confined disease: pT2a versus pT2b. Cancer. 2004; 100:1646–1649. PMID:
15073852.

15. Chun FK, Briganti A, Lebeau T, Fradet V, Steuber T, Walz J, et al. The 2002 AJCC pT2 substages confer no prognostic information on the rate of biochemical recurrence after radical prostatectomy. Eur Urol. 2006; 49:273–278. discussion 278–9. PMID:
16413103.

16. Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974; 111:58–64. PMID:
4813554.

17. Brierley JD, Gospodarowicz MK, O'Sullivan B, Wittekind C. TNM classification of malignant tumours. 8th ed. Chichester: Wiley-Blackwell;2017.
18. Kordan Y, Chang SS, Salem S, Cookson MS, Clark PE, Davis R, et al. Pathological stage T2 subgroups to predict biochemical recurrence after prostatectomy. J Urol. 2009; 182:2291–2295. PMID:
19758638.

19. Caso JR, Tsivian M, Mouraviev V, Polascik TJ, Moul JW. Pathological T2 sub-divisions as a prognostic factor in the biochemical recurrence of prostate cancer. BJU Int. 2010; 106:1623–1627. PMID:
20553260.

20. Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, et al. Should pathologists routinely report prostate tumour volume? The prognostic value of tumour volume in prostate cancer. Eur Urol. 2010; 57:821–829. PMID:
19664875.

21. Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002; 60:264–269. PMID:
12137824.

22. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974. PMID:
9749478.
23. D'Amico AV, Cote K, Loffredo M, Renshaw AA, Chen MH. Pretreatment predictors of time to cancer specific death after prostate specific antigen failure. J Urol. 2003; 169:1320–1324. PMID:
12629352.
24. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D'amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008; 179:1354–1360. discussion 1360–1. PMID:
18289596.

25. Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011; 185:869–875. PMID:
21239008.

26. Mortezavi A, Sulser T, Robbiani J, Drescher E, Disteldorf D, Eberli D, et al. Long-term oncologic outcome of an initial series of laparoscopic radical prostatectomy for clinically localized prostate cancer after a median follow-up of 10 years. Clin Genitourin Cancer. 2016; 14:290–297. PMID:
26710661.

27. Isbarn H, Wanner M, Salomon G, Steuber T, Schlomm T, Köllermann J, et al. Long-term data on the survival of patients with prostate cancer treated with radical prostatectomy in the prostate-specific antigen era. BJU Int. 2010; 106:37–43. PMID:
20002667.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download