1. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141. PMID:
25761484.

2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID:
25220842.

3. Lee DH, Jung HB, Chung MS, Lee SH, Chung BH. The change of prostate cancer treatment in Korea: 5 year analysis of a single institution. Yonsei Med J. 2013; 54:87–91. PMID:
23225803.

4. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159. PMID:
18309951.

5. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–1512. PMID:
15470213.

6. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371:424–433. PMID:
24881730.

7. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187–1197. PMID:
22894553.

8. Saad F, de Bono J, Shore N, Fizazi K, Loriot Y, Hirmand M, et al. Efficacy outcomes by baseline prostate-specific antigen quartile in the AFFIRM trial. Eur Urol. 2015; 67:223–230. PMID:
25171902.

9. Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015; 16:509–521. PMID:
25888263.

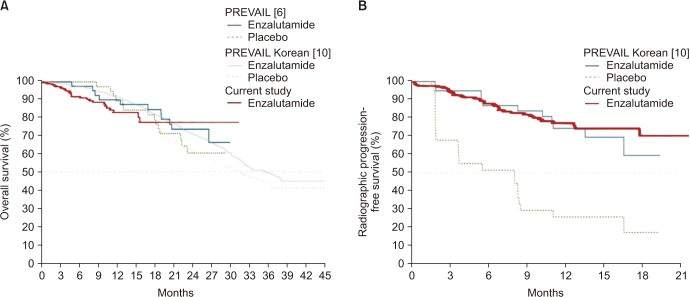
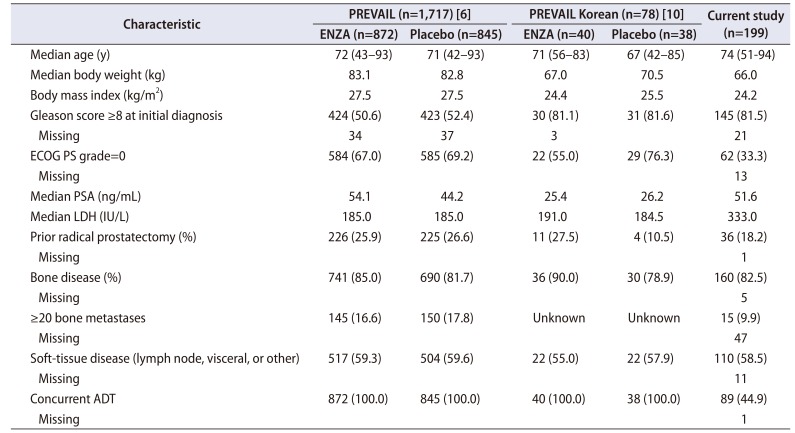
10. Kim CS, Theeuwes A, Kwon DD, Choi YD, Chung BH, Lee HM, et al. The PREVAIL trial of enzalutamide in men with chemotherapy-naïve, metastatic castration-resistant prostate cancer: post hoc analysis of Korean patients. Investig Clin Urol. 2016; 57:174–183.

11. Merseburger AS, Hammerer P, Rozet F, Roumeguère T, Caffo O, da Silva FC, et al. Androgen deprivation therapy in castrate-resistant prostate cancer: how important is GnRH agonist backbone therapy. World J Urol. 2015; 33:1079–1085. PMID:
25261259.

12. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. European Association of Urology. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479. PMID:
24321502.

13. Merseburger AS, Björk T, Whitehouse J, Meani D. Treatment costs for advanced prostate cancer using luteinizing hormone-releasing hormone agonists: a solid biodegradable leuprorelin implant versus other formulations. J Comp Eff Res. 2015; 4:447–453. PMID:
25521079.

14. Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993; 11:2167–2172. PMID:
8229130.

15. Hussain M, Wolf M, Marshall E, Crawford ED, Eisenberger M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol. 1994; 12:1868–1875. PMID:
8083710.

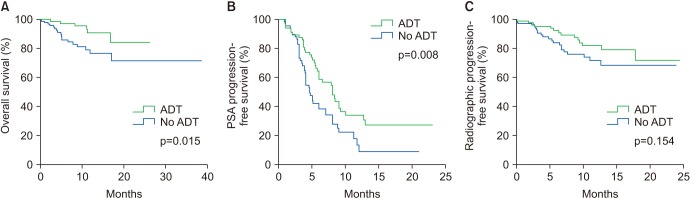
16. Lee JL, Eun Kim J, Ahn JH, Lee DH, Lee J, Kim CS, et al. Role of androgen deprivation treatment in patients with castration-resistant prostate cancer, receiving docetaxel-based chemotherapy. Am J Clin Oncol. 2011; 34:140–144. PMID:
20686407.

17. Bong GW, Clarke HS Jr, Hancock WC, Keane TE. Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology. 2008; 71:1177–1180. PMID:
18279929.

18. Kobayashi T, Nishizawa K, Mitsumori K. Individual variation of hormonal recovery after cessation of luteinizing hormone-releasing hormone agonist therapy in men receiving long-term medical castration therapy for prostate cancer. Scand J Urol Nephrol. 2006; 40:198–203. PMID:
16809259.

19. Kaku H, Saika T, Tsushima T, Ebara S, Senoh T, Yamato T, et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate. 2006; 66:439–444. PMID:
16329145.

20. Hall MC, Fritzsch RJ, Sagalowsky AI, Ahrens A, Petty B, Roehrborn CG. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology. 1999; 53:898–902. discussion 902–3. PMID:
10223480.

21. Pinski J, Xiong S, Wang Q, Stanczyk F, Hawes D, Liu SV. Effect of luteinizing hormone on the steroidogenic pathway in prostate cancer. Prostate. 2011; 71:892–898. PMID:
21456071.

22. O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004; 90:2317–2325. PMID:
15150570.
23. Ohlmann CH, Jäschke M, Jaehnig P, Krege S, Gschwend J, Rexer H, et al. Abiraterone acetate plus LHRH therapy versus abiraterone acetate while sparing LHRH therapy in patients with progressive, metastatic and chemotherapy-naïve, castration-resistant prostate cancer (SPARE): study protocol for a randomized controlled trial. Trials. 2017; 18:457. PMID:
28978327.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download