Abstract
There have been few studies investigating the association between atopic dermatitis (AD) and prenatal exposure to heavy metals. We aimed to evaluate whether prenatal exposure to heavy metals is associated with the development or severity of AD in a birth cohort study. A total of 331 subjects were followed from birth for a median duration of 60.0 months. The presence and severity of AD were evaluated at ages 6 and 12 months, and regularly once a year thereafter. The concentrations of lead, mercury, chromium, and cadmium in umbilical cord blood were measured by inductively coupled plasma mass spectrometry. Cord blood mononuclear cells (CBMCs) were isolated and stimulated for analysis of cytokine production using ELISA. Heavy metal levels in cord blood were not associated with the development of AD until 24 months of age. However, a positive correlation was observed between the duration of AD and lead levels in cord blood (p=0.002). AD severity was also positively associated with chromium concentrations in cord blood (p=0.037), while cord blood levels of lead, mercury, and cadmium were not significantly associated with AD severity (p=0.562, p=0.054, and p=0.055, respectively). Interleukin-13 production in CBMCs was positively related with lead and chromium levels in cord blood (p=0.021 and p=0.015, respectively). Prenatal exposure to lead and chromium is associated with the persistence and severity of AD, and the immune reaction toward a Th2 polarization.
Go to : 
References
1. Kim CH, Kim SH, Lee JS. Association of maternal depression and allergic diseases in Korean children. Allergy Asthma Proc. 2017; 38:300–308.

2. Yang HK, Choi J, Kim WK, Lee SY, Park YM, Han MY, Kim HY, Hahm MI, Chae Y, Lee KJ, et al. The association between hypovitaminosis D and pediatric allergic diseases: a Korean nationwide population-based study. Allergy Asthma Proc. 2016; 37:64–69.

3. Chung Y, Kwon JH, Kim J, Han Y, Lee SI, Ahn K. Retrospective analysis of the natural history of atopic dermatitis occurring in the first year of life in Korean children. J Korean Med Sci. 2012; 27:723–728.

5. Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014; 134:993–999.

6. Jedrychowski W, Perera F, Maugeri U, Miller RL, Rembiasz M, Flak E, Mroz E, Majewska R, Zembala M. Intrauterine exposure to lead may enhance sensitization to common inhalant allergens in early childhood: a prospective prebirth cohort study. Environ Res. 2011; 111:119–124.

7. Herbarth O, Fritz GJ, Rehwagen M, Richter M, Röder S, Schlink U. Association between indoor renovation activities and eczema in early childhood. Int J Hyg Environ Health. 2006; 209:241–247.

9. Kim NH, Hyun YY, Lee KB, Chang Y, Ryu S, Oh KH, Ahn C. Environmental heavy metal exposure and chronic kidney disease in the general population. J Korean Med Sci. 2015; 30:272–277.

10. Kim JH, Jeong KS, Ha EH, Park H, Ha M, Hong YC, Lee SJ, Lee KY, Jeong J, Kim Y. Association between prenatal exposure to cadmium and atopic dermatitis in infancy. J Korean Med Sci. 2013; 28:516–521.

11. Yang HJ, Lee SY, Suh DI, Shin YH, Kim BJ, Seo JH, Chang HY, Kim KW, Ahn K, Shin YJ, et al. The Cohort for Childhood Origin of Asthma and allergic diseases (COCOA) study: design, rationale and methods. BMC Pulm Med. 2014; 14:109.

12. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
13. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993; 186:23–31.
14. Kim HB, Ahn KM, Kim KW, Shin YH, Yu J, Seo JH, Kim HY, Kwon JW, Kim BJ, Kwon JY, et al. Cord blood cellular proliferative response as a predictive factor for atopic dermatitis at 12 months. J Korean Med Sci. 2012; 27:1320–1326.

15. Mishra KP. Lead exposure and its impact on immune system: a review. Toxicol In Vitro. 2009; 23:969–972.

16. García-Esquinas E, Pérez-Gómez B, Fernández-Navarro P, Fernández MA, de Paz C, Pérez-Meixeira AM, Gil E, Iriso A, Sanz JC, Astray J, et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health. 2013; 13:841.

17. Schneider BC, Constant SL, Patierno SR, Jurjus RA, Ceryak SM. Exposure to particulate hexavalent chromium exacerbates allergic asthma pathology. Toxicol Appl Pharmacol. 2012; 259:38–44.

18. Zheng G, Zhong H, Guo Z, Wu Z, Zhang H, Wang C, Zhou Y, Zuo Z. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biol Trace Elem Res. 2014; 160:437–444.

19. Liu Y, Chen Q, Wei X, Chen L, Zhang X, Chen K, Chen J, Li T. Relationship between perinatal antioxidant vitamin and heavy metal levels and the growth and cognitive development of children at 5 years of age. Asia Pac J Clin Nutr. 2015; 24:650–658.
20. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc B. 2000; 62:711–730.

21. Miller TE, Golemboski KA, Ha RS, Bunn T, Sanders FS, Dietert RR. Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicol Sci. 1998; 42:129–135.

22. Dietert RR, Piepenbrink MS. Perinatal immunotoxicity: why adult exposure assessment fails to predict risk. Environ Health Perspect. 2006; 114:477–483.

23. Bunn TL, Dietert RR, Ladics GS, Holsapple MP. Developmental immunotoxicology assessment in the rat: age, gender, and strain comparisons after exposure to lead. Toxicol Methods. 2001; 11:41–58.

25. Lee SC. Committee of Korean Atopic Dermatitis Association for REACH. Various diagnostic criteria for atopic dermatitis (AD): a proposal of Reliable Estimation of Atopic Dermatitis in Childhood (REACH) criteria, a novel questionnaire-based diagnostic tool for AD. J Dermatol. 2016; 43:376–384.

26. Ashley-Martin J, Levy AR, Arbuckle TE, Platt RW, Marshall JS, Dodds L. Maternal exposure to metals and persistent pollutants and cord blood immune system biomarkers. Environ Health. 2015; 14:52.

27. Hon KL, Wang SS, Hung EC, Lam HS, Lui HH, Chow CM, Ching GK, Fok TF, Ng PC, Leung TF. Serum levels of heavy metals in childhood eczema and skin diseases: friends or foes. Pediatr Allergy Immunol. 2010; 21:831–836.

28. Min KB, Min JY. Environmental lead exposure and increased risk for total and allergen-specific IgE in US adults. J Allergy Clin Immunol. 2015; 135:275–277.

29. Gao D, Kasten-Jolly J, Lawrence DA. The paradoxical effects of lead in interferon-gamma knockout BALB/c mice. Toxicol Sci. 2006; 89:444–453.

30. Sughis M, Nawrot TS, Haufroid V, Nemery B. Adverse health effects of child labor: high exposure to chromium and oxidative DNA damage in children manufacturing surgical instruments. Environ Health Perspect. 2012; 120:1469–1474.

31. Strenzke N, Grabbe J, Plath KE, Rohwer J, Wolff HH, Gibbs BF. Mercuric chloride enhances immunoglobulin E-dependent mediator release from human basophils. Toxicol Appl Pharmacol. 2001; 174:257–263.

32. Ban M, Langonné I, Goutet M, Huguet N, Pépin E. Simultaneous analysis of the local and systemic immune responses in mice to study the occupational asthma mechanisms induced by chromium and platinum. Toxicology. 2010; 277:29–37.

Go to : 
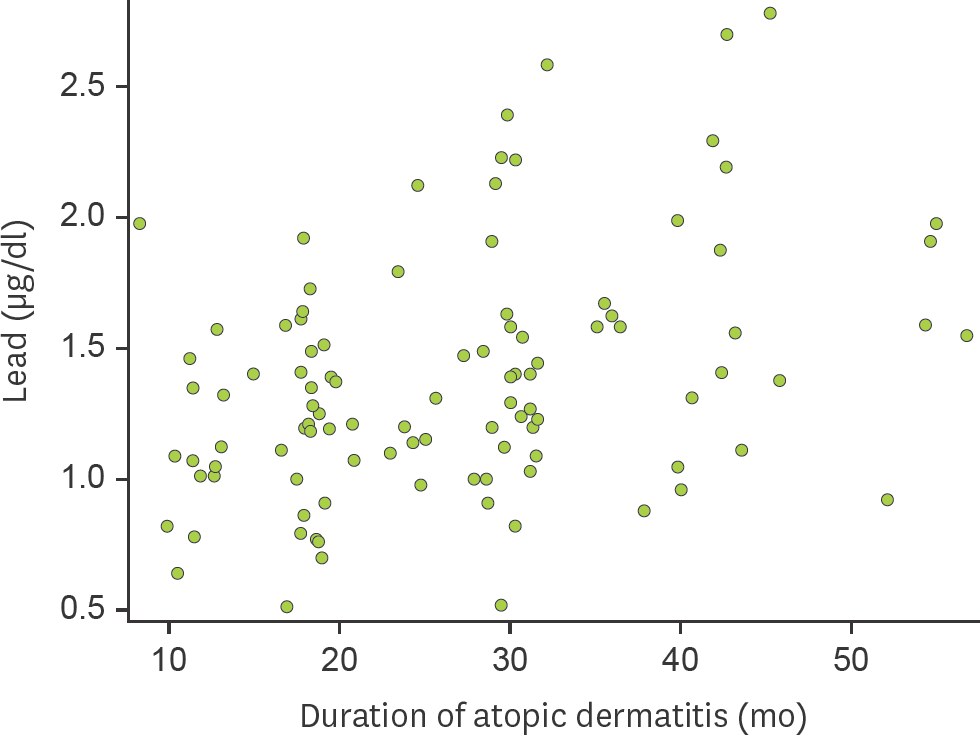 | Figure 1.Correlation between lead levels in cord blood and the duration of AD in 103 children whose skin symptoms lasted for more than 6 months. Statistical analysis was done using partial Spearman's correlation analysis after adjustment for gender, presence of siblings, season of birth, and antibiotic treatment during the first 6 months of life (ρ=0.308, p=0.002). |
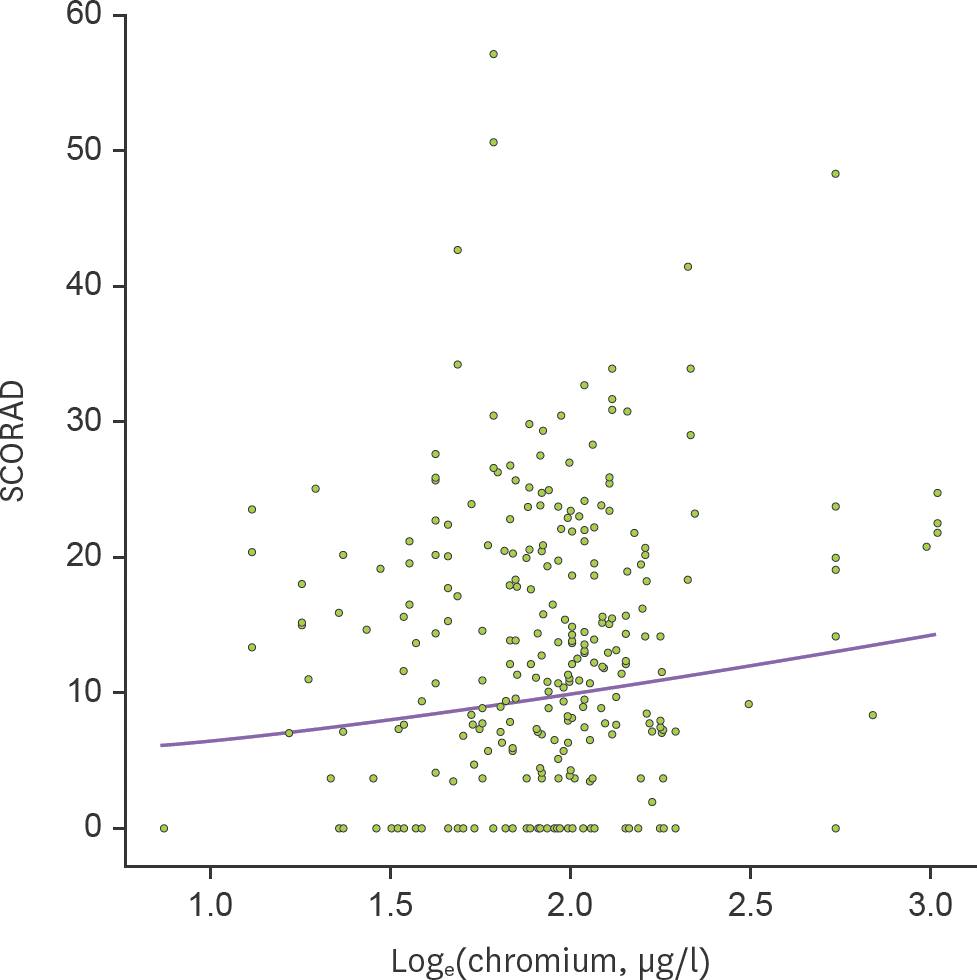 | Figure 2.Association between mercury levels in cord blood and SCORAD in children with AD. Mixed model was applied after adjustment for season of birth (p=0.004). |
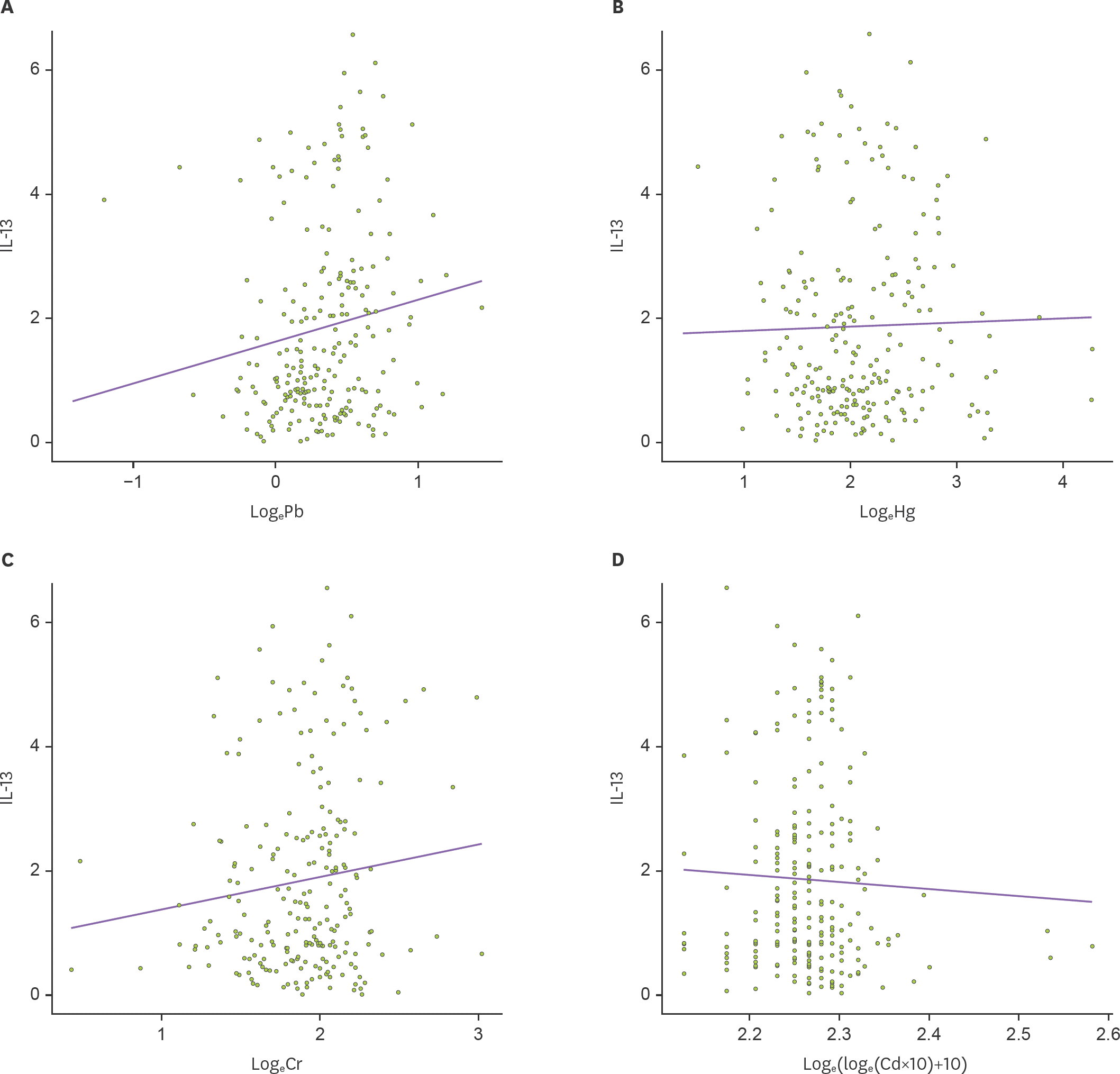 | Figure 3.Relationship between heavy metal levels and IL-13 in umbilical cord blood. Mixed model was applied to analyze the association of IL-13 with (A) lead (Pb, µg/dl), (B) mercury (Hg, µg/l), (C) chromium (Cr, µg/l), and (D) cadmium (Cd, µg/l). IL-13 levels in cord blood was positively correlated with only lead levels in cord blood (p=0.028). |
Table 1.
Characteristics of subjects (n=331)
Table 2.
Univariable and multivariable analyses for factors influencing the development of AD
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Lead* | 0.995 | 0.626–1.580 | 0.956 | 0.598–1.529 |
| Mercury* | 0.972 | 0.730–1.294 | 0.981 | 0.726–1.324 |
| Chromium* | 1.455 | 0.889–2.380 | 1.449 | 0.883–2.376 |
| Cadmium† | 3.503 | 0.250–49.083 | 2.525 | 0.197–32.419 |
Table 3.
Relationship between heavy metal levels in umbilical cord blood and SCORAD* index in children with AD
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Beta coefficient | p value | Beta coefficient | p value | |
| Lead* | 0.003 | 0.123 | 1.113 | 0.562 |
| Mercury* | 0.115 | 0.072 | 2.179 | 0.054 |
| Chromium* | 0.260 | 0.135 | 0.277 | 0.037 |
| Cadmium† | 1.524 | 0.855 | 25.258 | 0.055 |
Table 4.
Relationship between IL-13 and heavy metal levels in umbilical cord blood
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Beta coefficient | p value | Beta coefficient | p value | |
| Lead* | 0.676 | 0.028 | 0.692 | 0.021 |
| Mercury* | 0.067 | 0.726 | 0.102 | 0.585 |
| Chromium* | 0.530 | 0.075 | 0.713 | 0.015 |
| Cadmium† | −1.139 | 0.520 | −0.388 | 0.824 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download