Abstract
We previously demonstrated that atherogenic Ldlr−/− Apobec1−/− (LDb) double knockout mice lacking both low-density lipoprotein receptor (LDLR) and apolipoprotein B mRNA-editing catalytic polypeptide-1 (Apobec1) had increased serum IL-17 levels, with T cell programming shifted towards Th17 cells. In this study, we assessed the role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in T cell programming and atherogenesis. We deleted the Pcsk9 gene from LDb mice to generate Ldlr−/− Apobec1−/− Pcsk9−/− (LTp) triple knockout mice. Atherosclerosis in the aortic sinus and aorta were quantitated. Lymphoid cells were analyzed by flow cytometry, ELISA and real-time PCR. Despite of dyslipidemia, LTp mice developed barely detectable atherosclerotic lesions. The IL-17, was very low in plasma and barely detectable in the aortic sinus in the LTp mice. In the spleen, the number of CD4+ CD8− cells and splenocytes were much lower in the LDb mice than LTp mice, whereas, the IL-17-producing cells of γδ TCR+ T cells and effector memory CD4+ T cells (CD44 hi CD4+) in the spleen were significantly higher in the LDb mice than in the LTp mice. The Rorc mRNA expression levels were elevated in LDb mice compared to LTp mice. When re-stimulated with an anti-CD3 Ab, CD44 hi CD4+ T cells from LDb mice secreted more IL-17 than those from LTp mice. T cells from LDb mice (with PCSK9) produce more IL-17 at basal and stimulated conditions when compared with LTp mice (without PCSK9). Despite the dyslipidemic profile and the lack of LDLR, atherogenesis is markedly reduced in LTp mice. These results suggest that PCSK9 is associated with changes in T cell programming that contributes to the development of atherosclerosis.
References
1. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011; 473:317–325.

2. Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012; 16:1978–1990.
3. Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992; 1:445–466.

4. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013; 38:1092–1104.

6. Wong BW, Meredith A, Lin D, McManus BM. The biological role of inflammation in atherosclerosis. Can J Cardiol. 2012; 28:631–641.

7. Lim H, Kim YU, Sun H, Lee JH, Reynolds JM, Hanabuchi S, Wu H, Teng BB, Chung Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014; 40:153–165.

8. Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012; 32:273–280.

9. Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009; 119:1424–1432.

10. Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009; 183:8167–8175.

11. Gisterå A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013; 5:196ra100.

12. Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009; 206:2067–2077.

13. Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc Natl Acad Sci U S A. 2005; 102:2069–2074.

14. Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004; 279:50630–50638.

15. Sun H, Krauss RM, Chang JT, Teng BB. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J Lipid Res. 2018; 59:207–223.

16. Sun H, Samarghandi A, Zhang N, Yao Z, Xiong M, Teng BB. Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor. Arterioscler Thromb Vasc Biol. 2012; 32:1585–1595.

17. Daugherty A, Lu H, Howatt DA, Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol. 2009; 573:1–15.

18. Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005; 36:129–138.

19. Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987; 68:231–240.

20. Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995; 36:2320–2328.

21. Dutta R, Singh U, Li TB, Fornage M, Teng BB. Hepatic gene expression profiling reveals perturbed calcium signaling in a mouse model lacking both LDL receptor and Apobec1 genes. Atherosclerosis. 2003; 169:51–62.

22. Mak S, Sun H, Acevedo F, Shimmin LC, Zhao L, Teng BB, Hixson JE. Differential expression of genes in the calcium-signaling pathway underlies lesion development in the LDb mouse model of atherosclerosis. Atherosclerosis. 2010; 213:40–51.

23. Singh U, Zhong S, Xiong M, Li TB, Sniderman A, Teng BB. Increased plasma non-esterified fatty acids and platelet-activating factor acetylhydrolase are associated with susceptibility to atherosclerosis in mice. Clin Sci (Lond). 2004; 106:421–432.

24. Nischal H, Sun H, Wang Y, Ford DA, Cao Y, Wei P, Teng BB. Long-term expression of apolipoprotein B mRNA-specific hammerhead ribozyme via scAAV8.2 vector inhibits atherosclerosis in mice. Mol Ther Nucleic Acids. 2013; 2:e125.

25. Liu A, Frostegård J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J Intern Med. 2018; 284:193–210.

26. Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013; 2:e60.

27. Stepanova H, Mensikova M, Chlebova K, Faldyna M. CD4+ and γδ TCR+ T lymphocytes are sources of interleukin-17 in swine. Cytokine. 2012; 58:152–157.

28. Kysenius K, Muggalla P, Mätlik K, Arumäe U, Huttunen HJ. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell Mol Life Sci. 2012; 69:1903–1916.

29. Canuel M, Sun X, Asselin MC, Paramithiotis E, Prat A, Seidah NG. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS One. 2013; 8:e64145.

30. Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Commun. 2008; 375:69–73.

31. Demers A, Samami S, Lauzier B, Des Rosiers C, Ngo Sock ET, Ong H, Mayer G. PCSK9 induces CD36 degradation and affects long-chain fatty acid uptake and triglyceride metabolism in adipocytes and in mouse liver. Arterioscler Thromb Vasc Biol. 2015; 35:2517–2525.

32. Labonté P, Begley S, Guévin C, Asselin MC, Nassoury N, Mayer G, Prat A, Seidah NG. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology. 2009; 50:17–24.

33. Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. 2012; 287:19266–19274.

34. Proto JD, Doran AC, Subramanian M, Wang H, Zhang M, Sozen E, Rymond CC, Kuriakose G, D'Agati V, Winchester R, et al. Hypercholesterolemia induces T cell expansion in humanized immune mice. J Clin Invest. 2018; 128:2370–2375.

35. Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity. 2017; 47:621–634.

36. Momtazi-Borojeni AA, Jaafari MR, Badiee A, Sahebkar A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis. 2019; 283:69–78.

37. Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010; 1183:211–221.
38. Hilvo M, Simolin H, Metso J, Ruuth M, Öörni K, Jauhiainen M, Laaksonen R, Baruch A. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis. 2018; 269:159–165.

39. Adam D, Heinrich M, Kabelitz D, Schütze S. Ceramide: does it matter for T cells? Trends Immunol. 2002; 23:1–4.
40. Surls J, Nazarov-Stoica C, Kehl M, Olsen C, Casares S, Brumeanu TD. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS One. 2012; 7:e38733.

41. Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016; 531:651–655.

42. Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007; 117:1119–1127.

43. Krauzová E, Krač merová J, Rossmeislová L, Mališová L, Tencerová M, Koc M, Štich V, Šiklová M. Acute hyperlipidemia initiates proinflammatory and proatherogenic changes in circulation and adipose tissue in obese women. Atherosclerosis. 2016; 250:151–157.

44. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009; 27:485–517.

45. Mai J, Nanayakkara G, Lopez-Pastrana J, Li X, Li YF, Wang X, Song A, Virtue A, Shao Y, Shan H, et al. Interleukin-17A promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (MAPK) pathway. J Biol Chem. 2016; 291:4939–4954.

46. Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010; 121:1746–1755.

47. Li G, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M, Hisamitsu T, Liu Y. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κ B/HIF-1α pathway. Mol Immunol. 2013; 53:227–236.

48. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003; 101:2620–2627.

49. Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, Haller H, von Vietinghoff S. Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor a and are normalized by inhibition of interleukin-17A. JACC Basic Transl Sci. 2018; 3:54–66.

50. Billon C, Sitaula S, Burris TP. Inhibition of RORα/γ suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metab. 2016; 5:997–1005.

51. Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011; 215:471–474.

52. Ge S, Hertel B, Koltsova EK, Sörensen-Zender I, Kielstein JT, Ley K, Haller H, von Vietinghoff S. Increased atherosclerotic lesion formation and vascular leukocyte accumulation in renal impairment are mediated by interleukin-17A. Circ Res. 2013; 113:965–974.

53. Brauner S, Jiang X, Thorlacius GE, Lundberg AM, Östberg T, Yan ZQ, Kuchroo VK, Hansson GK, Wahren-Herlenius M. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc Res. 2018; 114:158–167.

54. Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005; 102:1596–1601.

55. Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation. 2018; 138:1130–1143.

56. Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016; 13:167–179.

57. Proto JD, Doran AC, Gusarova G, Yurdagul A Jr, Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018; 49:666–677. e6.

58. Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012; 110:675–687.

59. Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018; 9:1095.

Figure 1.
The effect of PCSK9 on atherosclerosis development and on the levels of plasma cholesterol and triglyceride in LDb and LTp male mice at 5 months of age. (A) The atherosclerotic lesions using en face quantification method on the aorta of LDb (n=10) and LTp (n=10) mice are expressed as % lesions, which were calculated as the ratio of aortic surface covered by plaques (in square millimeters) divided by the total surface of the whole aorta (in square millimeters). The results of % plaques of each aorta and mean±SD are shown. (B) The plaque area (in square microns) of each aortic sinus (LDb=5 and LTp=6) were quantified using AxioVision release 4.8 software (Zeiss). Each data point represents an average of 6 sections of aortic sinus for 1 animal. The plaque size (µm2) of each animal and mean±SD are shown. We used 2-tailed unpaired t-test with Welch's correction to analyze the difference between LDb versus LTp mice. The p values are shown. (C) The concentrations (mg/dl) of plasma cholesterol and triglyceride in C57BL/6J (n=7), LDb(n=7), and LTp (n=7) are presented. The mean±SD of each group is shown. Statistical analysis were performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. One-way ANOVA was also used to analyze the results.
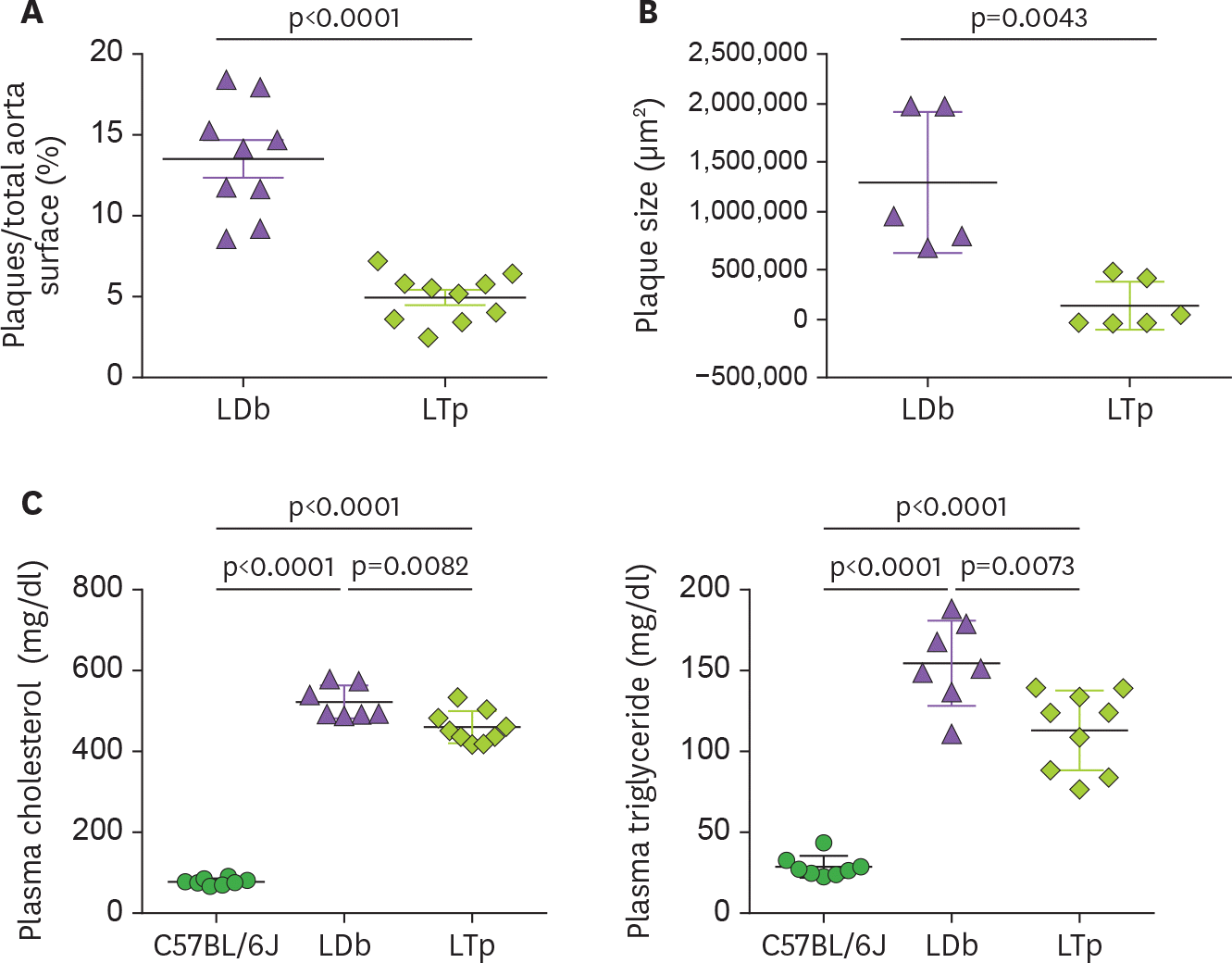
Figure 2.
Analysis of T cell in the thymus and the spleen of C57BL/6J, LDb and LTp mice. (A) A representative result of flow cytometry analysis showing the frequencies of T cell populations in the thymus of WT (C57BL/6J), LDb and LTp mice is shown. (B) The frequencies of CD4+ CD8− T cells from the thymus of WT (C57BL/6J), LDb and LTp mice (male mice at 5-months of age, n=6 for each strain) were analyzed by flow cytometry. The results of the analyses and mean±SD are shown. (C) The total number of splenocytes in the spleen of WT (C57BL/6J), LDb and LTp mice (male mice at 5-months of age, n=6 for each strain) were analyzed by flow cytometry. The results of the analyses and mean±SD are shown. (D) The total number of CD4+ T cells in the spleen of WT (C57BL/6J), LDb and LTp mice (male mice at 5-months of age, n=6 for each strain) were analyzed by flow cytometry. The results of the analyses and mean±SD are shown. Statistical analysis of results was performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. The p<0.05 is considered significantly. ns, not significant.
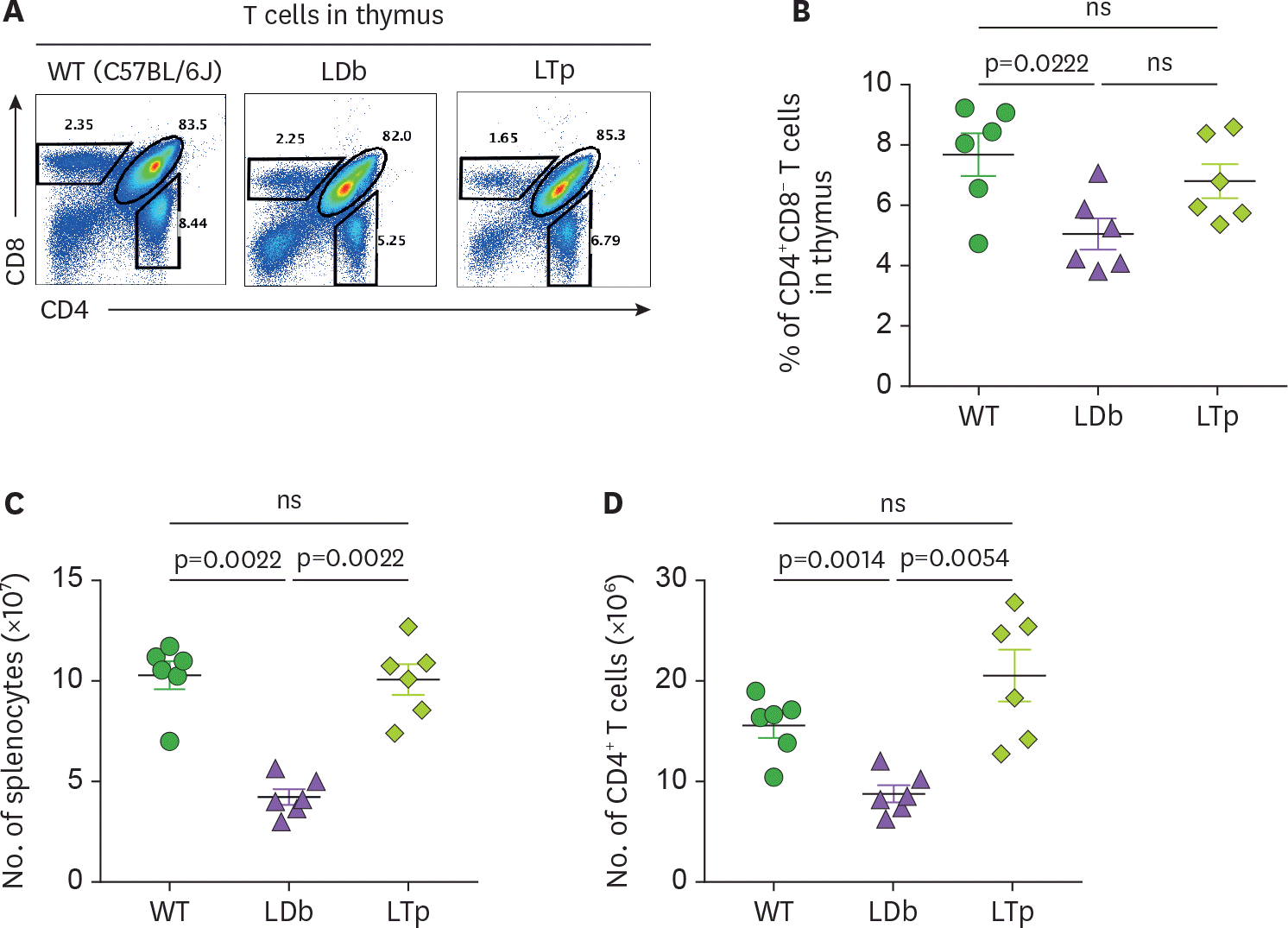
Figure 3.
The effect of PCSK9 on IL-17 in C57BL/6J (WT), LDb and LTp mice. (A) LTp mice had barely detectable plasma levels of IL-17. IL-17 levels in serum obtained from C57BL/6J (n=4), LDb (n=5), and LTp (n=5) mice were measured by ELISA and expressed as pg/ml. (B) LTp mice had barely detectable expression of IL-17 in the aortic sinus. Aortic sinus sections obtained from LDb (n=6) and LTp (n=6) mice were stained with isotype control or polyclonal anti-IL-17 (red) and DAPI (blue). We quantified the intensity of immunofluorescence images expressing IL-17 from each section. LDb mice show increased expression of IL-17 in the aortic sinus, compared to LTp mice. The densities of the expressions of IL17 were determined. (C). LTp mice had decreased numbers of γδ TCR+ T cells, compared to LDb mice. The absolute numbers of γδ TCR+ T cells in the spleen of WT (C57BL/6J, n=6), LDb (n=6), and LTp (n=6) mice were analyzed by flow cytometry. (D) LTp mice had decreased % of γδ TCR+ T cells, compared to LDb mice. The frequency of γδ TCR+ T cells in the spleen of WT (C57BL/6J, n=6), LDb (n=6), and LTp (n=6) mice were analyzed by flow cytometry. All results are presented as mean±SD in this figure. Statistical analysis of the esults was performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. The p<0.05 is considered significantly. ns, not significant.
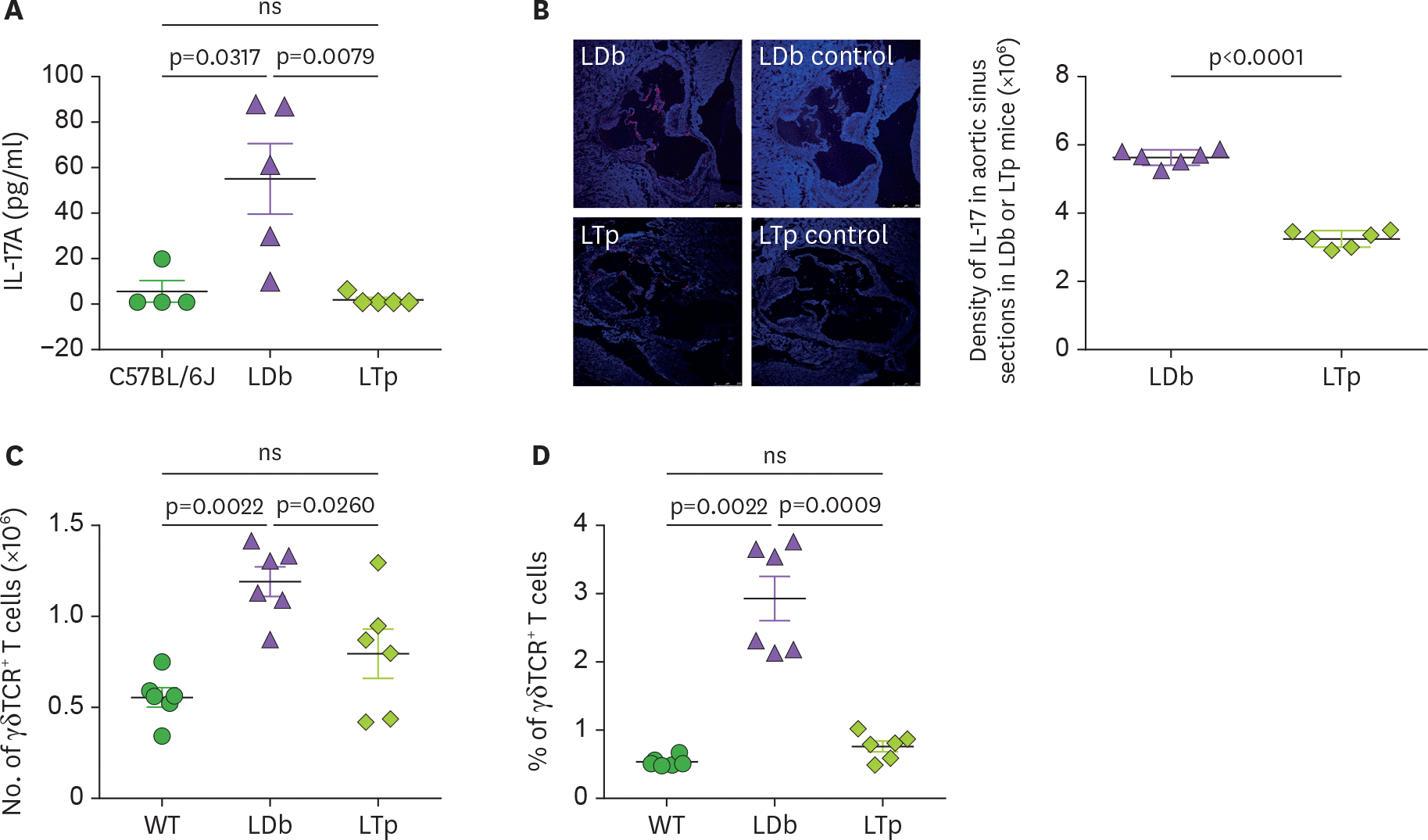
Figure 4.
Deficiency of PCSK9 reduces the expression and secretion of IL-17 from Th subset cells (CD44 hi CD4+) obtained from the spleens of C57BL/6J (WT), LDb, and LTp mice. (A) The frequencies of CD44 hi CD4+ T cells from the spleen of WT (C57BL/6J), LDb, and LTp mice were analyzed using flow cytometry. The results of the frequencies of CD44 hi CD4+ T cells and mean±SD are shown on the right. (B) The frequencies of IFN-γ and IL-17 expressing cells from CD44 hi CD4+ T cells in the spleen of WT (C57BL/6J, n=6), LDb (n=6), and LTp (n=6) mice are analyzed by flow cytometry. The frequencies and total number of IFN-γ and IL-17 expressing cells from CD44 hi CD4+ T cells are presented and mean±SD are also shown. (C) The relative expression levels of mRNA transcripts of Rorc and Tbx21 genes from the flow cytometry sorted CD44 hi CD4+ T cells of the spleen of WT (C57BL/6J), LDb, and LTp mice (n=6 from each strain) were determined using real-time qPCR. The sorted CD44 hi CD4+ T cells were stimulated with anti-CD3 (1 µg/ml) for 4 h, followed by RNA extraction and qPCR determination. The RNA levels from each animal and the mean±SD are shown. Statistical analyses of results in figures A to C was performed using 2-tailed unpaired t-tests with Welch's correction. The p values are listed. The p<0.05 is considered significantly. ns, not significant. (D) Re-stimulated CD44 hi CD4+ T cells from LDb mice secret more IL-17. The flow cytometry sorted CD44 hi CD4+ T cells (2×105 cells) from the spleen of WT (C57BL/6J), LDb, and LTp mice were re-stimulated with different concentrations of anti-CD3 (0, 0.5, and 5 µg/ml) for 3 days. The IL-17 (ng/ml) secreted to the media was measured using ELISA. The results are presented as mean±SD at each concentration. Data represents 3 independent experiments. Statistical analysis was performed using 2-way ANOVA. The p values are listed. ns, not significant.
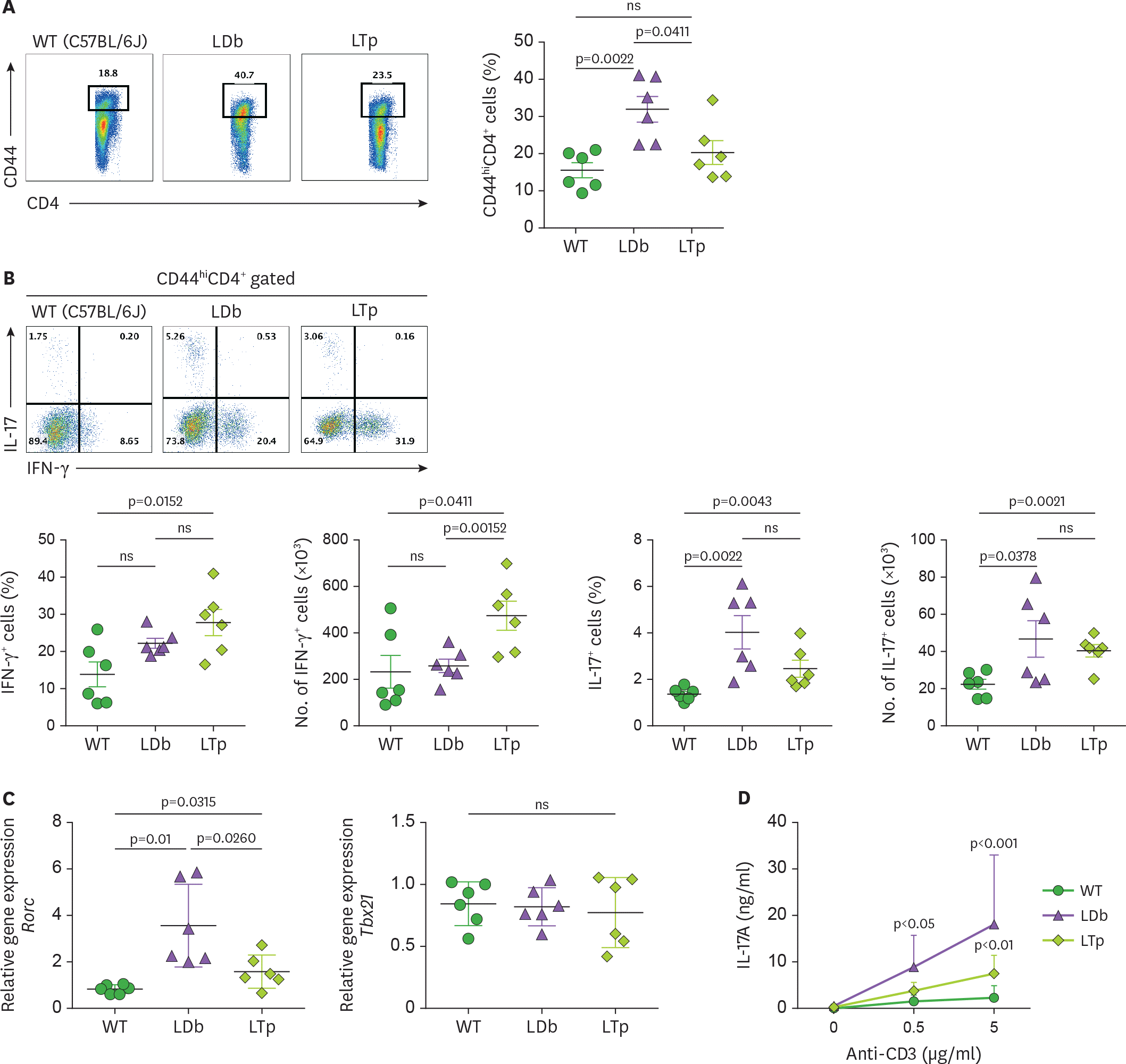
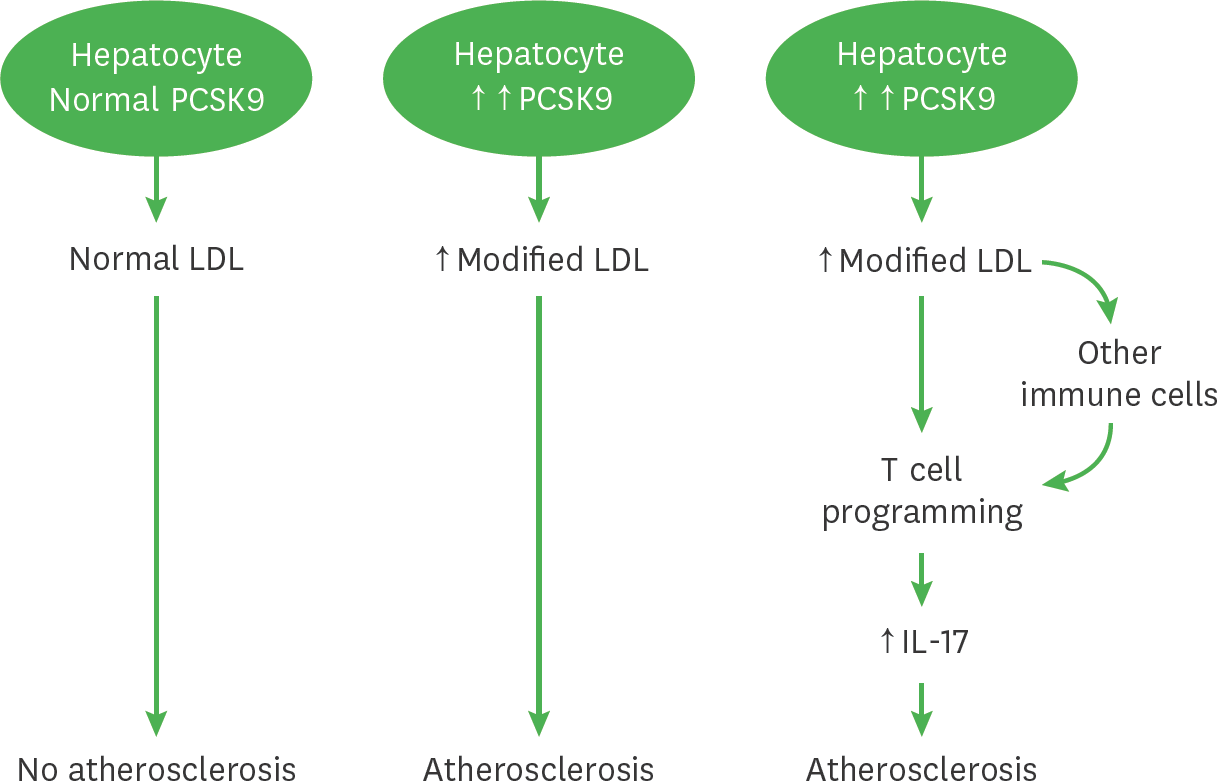
Figure 5.
A schematic diagram illustrates the role of PCSK9 mediates atherosclerosis via immune cells. This diagram displays the role of PCSK9 in atherosclerosis and immune response mediated via IL-17 cells and other immune cells including Th1, Treg and Tfh cells. Under normal lipidemic condition with low PCSK9 levels, the hepatocytes produce and secrete normal LDLs, these LDLs do not influence the development of atherosclerosis. Under hyperlipidemia condition with increased PCSK9 levels, the hepatocytes produce increased amounts of modified LDLs, which are cholesterol ester and phospholipid enriched. These modified LDLs contribute to the development of atherosclerosis by possibly altering T cells programing shifted towards IL-17 producing T cells to increase IL-17 production or via induce cytokines production to modulating immune cells including Th1, Treg or Tfh T cells to influence IL-17 production. Increased IL-17 contributes to the development of atherosclerosis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download