INTRODUCTION
MATERIALS AND METHODS
Mice
Disease induction and evaluation
FACS analysis
Histopathologic examination
ELISA and ELISPOT assay
Microfluorometry
Amylase assay
RESULTS AND DISCUSSION
SKG mice exhibit xerostomia upon systemic exposure to curdlan.
 | Figure 1Arthritis and xerostomia of curdlan-injected SKG mice. SKG mice were injected with curdlan or PBS. (A-C) Joint-draining LNs (B) and cLNs (A and C) at weeks 3 and 8 post-injection were assayed by FACS. Representative FACS profiles gated on CD4+ cells and percentages and numbers of IL-17+IFNγ− CD4+ and IL-17−IFNγ+ CD4+ cells are shown. (D) Ankle thickness and arthritic indexes were measured every week (n=3–4/group). (E) Mice were injected with pilocarpine, and saliva was collected at the indicated time. Graphs display means±SEMs (B-D) or ±SDs (E) with symbols representing the values of individual mice. All data are representative of more than 3 independent experiments.NS, not significant.
*p<0.05, **p<0.01, ***p<0.001 by 2-way ANOVA (D) and Student's t-test (B, C, and E).
|
Salivary glands from curdlan-treated SKG mice contain IgG deposits without focal leukocyte infiltrates
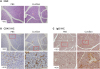 | Figure 2IgG deposition without immune cell infiltration in salivary glands from curdlan-injected SKG mice. SKG mice were injected with curdlan or PBS and subjected to histopathologic examination 8 weeks later. Tissue sections of salivary glands were stained with H&E (A), anti-CD45 Ab (B), and anti-IgG Ab (C). Photographs are representative of more than 3 individuals. Original magnifications are ×100 and ×400. The boxed areas were magnified in ×400 images.IHC, immunohistochemistry.
|
Serum titers of autoantibodies specific for salivary glands are elevated in curdlan-injected SKG mice
 | Figure 3Features of autoantibody production in curdlan-injected SKG mice. SKG mice were injected with curdlan or PBS. (A-E) Serum was collected at the indicated times and assayed by ELISA to measure titers of total IgG (A), anti-SGE IgG (B), anti-SSB IgG (C), anti-M3R IgG (D), and subclasses of anti-M3R IgG (E). (F) Spleen, cLNs and BM were harvested 8 weeks post-injection, and assayed by ELISPOT to enumerate anti-M3R Ab-secreting cells per 2×105 cells. Graphs display means±SEMs with or without symbols representing the values of individual mice.Data are pools of 3 (A-D, n=6–8 per group) or 2 (F) independent experiments, and representative of 2 independent experiments (E).
AU, arbitrary units; NS, not significant; BM, bone marrow; SGE, salivary gland extract.
*p<0.05, **p<0.01, ***p<0.001 by Student's t-test.
|
Serum from curdlan-injected SKG mice inhibits M3R-mediated Ca2+ signaling in salivary gland cells
 | Figure 4Inhibition of M3R-mediated Ca2+ signaling by serum from curdlan-injected SKG mice in salivary gland cells. (A-D) Submandibular salivary gland cells were pretreated with serum from PBS- or curdlan-injected SKG mice for 90 min. The cells were loaded with fura-2, perfused with CCh for 15 s (A and B) or 2 min (C and D), and assayed by microfluorometry. (A) Representative graphs displaying the F340/380 values of 3 cells. Values of Δ(F340/F380) are indicated by arrows. (B) Percentages of cells showing each range of Δ(F340/F380). Data are displayed as means±SEM of 2–3 independent experiments. (C) Representative profiles of Ca2+ oscillating waves. (D) Numbers of oscillating waves. The data are representative of 2–3 independent experiments. (E) Parotid salivary glands were pretreated with serum from PBS- or curdlan-injected SKG mice and assayed to measure amylase activity (mU/ml) per wet weight (mg) of the gland (n=5 per group pooled from 2 independent experiments).CCh, carbachol.
*p<0.05 and **p<0.01 by Student's t-test.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download