Invasive fungal diseases (IFDs) are a leading cause of morbidity and infection-related mortality among allogeneic hematopoietic stem cell transplantation (HSCT) recipients [
12]. In recent years, advanced diagnostic tests have considerably improved the diagnosis of IFD, but the standard methods often fail to timely detect the fungal pathogen and are not suitable for regular monitoring of an infection [
3]. The routine evaluation with chest radiography has several limitations due to the following: low sensitivity, patient condition, time consumption, and inter-observer variability in its interpretation [
45]. On the contrary, computed tomography (CT) is considered the gold standard technique, but may not be available in the due time; it involves a high radiation dose and relatively high cost [
6]. The lungs are considered poorly accessible to ultrasound (US) investigation because of their air content. Only in the last decade, it has been shown that the US assessment of the lung could have clinical implications [
7]. The lung US is increasingly applied owing to its application in rapid diagnosis and management of critically ill patients [
8]. The purpose of this study was to investigate the application of lung US in the evaluation of IFD in hematological patients undergoing allogeneic HSCT.
In this prospective non-interventional study, bedside lung US examination was longitudinally performed in patients with IFD undergoing allogeneic HSCT, at diagnosis and 10 days after the start of antifungal treatment. This pilot study was approved by local ethical committee (IRB's audit number:103/INT/2015) and written informed consent was obtained from all patients. Anti-infectious prophylaxis and treatment were administered according to institutional guidelines, based on international recommendations.
Within an 18-month recruitment period, 10 consecutive patients who developed an IFD after allogeneic HSCT were included in this pilot study. All evaluable patients had an established diagnosis of IFD according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) definitions [
3]. Computed tomography scan was used to diagnose fungal pneumonia, following the radiological patterns defined by the EORTC/MSG definitions [
3].
Convex 3.5 to 5 MHz probes (Logiq F6 model, portable device; GE Healthcare, Milan, Italy) were used. Two skilled physicians performed all study examinations. At bedside, the probe was set perpendicular, oblique, and parallel to the ribs in the anterior, lateral, and posterior (lower and upper) thorax. Sitting position and lateral decubitus were used to scan the posterior chest wall. Each hemithorax was divided into five areas [
9]: 2 anterior, 2 lateral, and 1 posterior. The anterior chest wall was marked off from the parasternal line to the anterior axillary line. This zone was divided into an upper region (from the collarbone to the second-third intercostal space) and a lower region (from the third intercostal space to the diaphragm). Furthermore, the lateral area, from the anterior to the posterior axillary lines, was divided into upper and lower halves. Finally, the posterior zone was demarcated from the posterior axillary line to the paravertebral line.
The criterion to determine the echographic diagnosis of pneumonia was the finding of subpleural lung consolidation with evidence of static or dynamic air bronchograms. Vertically oriented “comet-tail” artifacts, B lines [
10], might be present. We used the dynamic US signs for the differential diagnosis of pneumonia with pulmonary atelectasis, characterized by a liver-like appearance of the lung with “lung pulse” (absence of lung sliding and a parallel course of air).
The patients (median age 44.5 y) underwent allogeneic HSCT for high-risk hematological malignancies (6 with acute leukemia, 1 with chronic myeloid leukemia, 1 with Hodgkin lymphoma, and 1 with non-Hodgkin lymphoma); 50% of the patients were not in complete remission during transplantation. Stem cell donors were family haploidentical (n = 6), human leucocyte antigen identical sibling (n = 2), and unrelated volunteer (n = 2). The stem cell source was T-cell replete peripheral blood stem cells. All patients received a treosulfan-based conditioning regimen and graft-versus-host disease prophylaxis with post-transplant cyclophosphamide and sirolimus [
1112].
Early after HSCT (before neutrophil engraftment), we documented 8 possible and 2 probable pulmonary IFDs (
Table 1). Posaconazole was used as the antifungal therapy in 2 cases, voriconazole in 2, isavuconazole in 2, and a combination of liposomal amphotericin plus azole in 4.
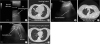
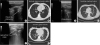
The lung US (
Fig. 1 and
Supplementary Fig. 1) was performed in all cases, concomitantly with high-resolution CT scan (
Fig. 2). A complete examination required 10 (10 - 15) min. The investigators judged examination conditions as “good” in all cases except one, which was considered “limited” due to poor patient compliance. The most important parenchymal US criterion was the positive air bronchogram within a hypoechoic area, which was found in 40% of the patients, and the most frequent pleural criterion was basal effusion (in 40% patients). At diagnosis, patients showed consolidations, hypoechoic areas, and an inhomogeneous echotexture with blurred margins, most frequently on both sides of the lungs (n = 7), rather than on the right (n = 1) or left (n = 2) side of the lungs.
Four patients presented a positive air bronchogram, reflecting residual air within consolidated areas and attesting bronchial patency, which ruled out obstructive atelectasis. Multiple lenticular echoes, representing air trapped in the smaller airways, were also observed in these patients. No fluid bronchogram was observed. Contiguous focal B-lines and a pleural line attenuation corresponding to the affected area were evident in eight patients. Pleural basal effusion was detected in four patients, appearing as an anechoic area in the pleural space.
During follow up, we documented a clinical improvement, which was also demonstrated by CT scan and lung US (
Fig. 1), in all patients except one. Pneumonic lesions appeared sonographically smaller and the volume of pleural effusion decreased in patients with a documented IFD response. Importantly, all US findings were comparable to the CT scan results (
Fig. 1 and
2). Correlations with CT images showed that B lines were related to the presence of interstitial syndrome [
7]. We did not observe any discrepancy in US findings between the two physicians.
Invasive fungal diseases continue to cause considerable morbidity and mortality in patients with hematological malignancy. Given the poor outcomes associated with IFD in transplant recipients, there is considerable interest in identifying early bedside diagnostic and prognostic tools that may help guide the development of tailored intervention strategies.
The lung US is considered a promising technique owing to its high sensitivity in the detection of lung lesions and in the follow-up examinations, with the advantages of being cost effective and time saving. The main advantages of bedside lung US include postponing or even avoiding transport of patients to the radiology unit (also preventing radiation exposure of CT scan) and guiding life-saving therapies in extreme emergencies. Most US devices are portable or hand held, and may be used in critical care situations and in patients in a semi-recumbent or supine position. However, the ability of US to detect general abnormalities in the lung rather than peculiar IFD signs as captured by CT scan, suggest US as a potential tool during the follow up of lung IFDs instead of its diagnosis.
The lung US was at least as good as X-ray evaluation in detecting pneumonia [
13]. Sonography can detect peripheral pneumonic lesions, even small ones, whereas X-ray covers central localized processes, but small ones may escape radiological detection. During the follow up, the lung US may appear normal because the lesions do not reach the peripheral pleura. Another possible limitation of US is the variation in its accuracy between different examiners, depending on the skill of the examiner. To overcome this variability, two skilled physicians performed all examinations in this study.
Moreover, the low rates of probable pulmonary IFDs and the predominance of possible IFDs in this study may have limited the application of lung US in hematological patients with IFDs. Nevertheless, the epidemiology of IFD in hematologic patients has significantly reduced during recent years, mainly due to mould-active antifungal prophylaxis [
14]. Moreover, diagnosis of IFD could be difficult in patients with neutropenia receiving mould-active antifungal prophylaxis; the clinical presentation of a pulmonary IFD may be influenced by the prophylaxis with increasing rate of atypical radiological findings non-fulfilling completely the EORTC-MSG criteria [
14]. We speculate that the number of probable/proven IFD could increase by conducting larger studies in the future and developing new diagnostic tools for the diagnosis of IFD, potentially unaffected by the use of mould-active antifungal prophylaxis. However, both techniques have advantages and disadvantages, and in conclusion, they are comparable as well as complementary.
Although limited by the small number of patients included in this pilot observational study, lung US could have a significant role in the diagnostic workup and even in the follow up of lung lesions in the onco-hematological area, considering it is a bedside, reliable, rapid, and non-invasive technique. In particular, if the accuracy of lung US can be confirmed in large trials, its prompt execution could be relevant for the critical care of patients, especially considering the time and cost required for radiologic surveys.
In conclusion, our preliminary study indicates that US techniques could be potentially relevant for a multidisciplinary IFD evaluation in this high-risk population.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download