Abstract
Purpose
Materials and Methods
Results
Conclusion
Figures and Tables
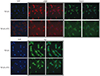
 | Fig. 1Recombinant adenovirus was transfected in fibroblast from the scar tissue of patients with adhesions around zone II flexor tendon after tendon repair by immunocytochemistry analysis, indicating highly efficient transduction rate of adenoviral marker gene construct when activated by transforming growth factor-beta 1 (TGF-β1). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. Ad-FST, adenovirus follistatin gene construct; DAPI, 4′,6-diamidino-2-phenylindole. |
 | Fig. 2Gene expressions of collagen I (A), collagen III (B), collagen IV (C), collagen V (D), and collagen XI (E) in fibroblast from adhesion tissue in patients with zone II flexor tendon repair and transfection with/without adenovirus follistatin gene construct (Ad-FST). Expression of collagen type I mRNA in fibroblast with Ad-FST showed a 25% decrease at 24 hours compared to control culture (TGF-β1+). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. *p<0.05. TGF-β1, transforming growth factor-beta 1; Ad-LacZ, adenovirus LacZ gene construct; SB505124, selective inhibitor of transforming growth factor-β type I receptor. |
 | Fig. 3Gene expressions of MMP-1 (A), MMP-2 (B), MMP-3 (C), MMP-9 (D), and MMP-13 (E) in fibroblast from adhesion tissue in patients with zone II flexor tendon repair and transfection with/without adenovirus follistatin gene construct (Ad-FST). Expressions of MMP-1 and -2 mRNA in fibroblast with Ad-FST showed a 31% and 59% decrease, respectively, at 24 hours compared to control culture (TGF-β1+). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. *p<0.05. TGF-β1, transforming growth factor-beta 1; MMP, matrix metalloproteinase; Ad-LacZ, adenovirus LacZ gene construct; SB505124, selective inhibitor of transforming growth factor-β type I receptor. |
 | Fig. 4Gene expressions of PAI-1 (A) and α-SMA (B) in fibroblast from adhesion tissue in patients with zone II flexor tendon repair and transfection with/without adenovirus follistatin gene construct (Ad-FST). Expression of α-SMA mRNA in the fibroblast with Ad-FST showed a 23% decrease at 24 hours compared to control culture (TGF-β1+). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. *p<0.05. TGF-β1, transforming growth factor-beta 1; Ad-LacZ, adenovirus LacZ gene construct; SB505124, selective inhibitor of transforming growth factor-β type I receptor. |
 | Fig. 5Protein expressions of MMPs (A), MMP-1 (B), MMP-2 (C), and MMP-13 (D) in fibroblast from adhesion tissue in patients with zone II flexor tendon repair and transfection with/without adenovirus follistatin gene construct (Ad-FST). Expression of MMP-1 protein in fibroblast with Ad-FST showed a 24% decrease at 24 hours compared to control culture (TGF-β1+). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. *p<0.05. TGF-β1, transforming growth factor-beta 1; MMP, matrix metalloproteinase; Ad-LacZ, adenovirus LacZ gene construct; SB505124, selective inhibitor of transforming growth factor-β type I receptor. |
 | Fig. 6Protein expressions of TIMP-1, -2, -4 (A–D) and fibronectin (E) in fibroblast from adhesion tissue in patients with zone II flexor tendon repair and transfection with/without adenovirus follistatin gene construct (Ad-FST). Expressions of TIMP-1 and fibronectin protein in fibroblast with Ad-FST showed a 23% and 24% decrease, respectively, at 24 hours compared to control culture (TGF-β1+). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. *p<0.05. TGF-β1, transforming growth factor-beta 1; Ad-LacZ, adenovirus LacZ gene construct; SB505124, selective inhibitor of transforming growth factor-β type I receptor. |
 | Fig. 7Protein expressions of TRPV4, PAI-1, and α-SMA (A) in fibroblast from adhesion tissue in patients with zone II flexor tendon repair and transfection with/without adenovirus follistatin gene construct (Ad-FST). Expressions of TRPV4 (B), PAI-1 (C), and α-SMA (D) protein in fibroblast with Ad-FST showed a 23%, 23%, and 28% decrease, respectively, at 24 hours compared to control culture (TGF-β1+). ‘+’ means ‘treatment’ and ‘−’ means ‘no treatment’. *p<0.05. TGF-β1, transforming growth factor-beta 1; Ad-LacZ, adenovirus LacZ gene construct; SB505124, selective inhibitor of transforming growth factor-β type I receptor. |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Young-Mi Kang, Su-Keon Lee, Yong-Min Chun, Seong-Hwan Moon, and Yun-Rak Choi.
Data curation: Young-Mi Kang, Su-Keon Lee, Yun-Rak Choi, and Seong-Hwan Moon.
Formal analysis: Young-Mi Kang, Su-Keon Lee, Yun-Rak Choi, and Seong-Hwan Moon.
Funding acquisition: Yun-Rak Choi.
Investigation: Young-Mi Kang, Su-Keon Lee, Yong-Min Chun, and Yun-Rak Choi.
Methodology: All authors.
Project administration: Yun-Rak Choi and Seong-Hwan Moon.
Resources: Yun-Rak Choi and Seong-Hwan Moon.
Software: Yun-Rak Choi, Seong-Hwan Moon, Hwan-Mo Lee, and Ho-Jung Kang.
Supervision: Seong-Hwan Moon, Hwan-Mo Lee, and Ho-Jung Kang.
Validation: Yun-Rak Choi, Seong-Hwan Moon, Hwan-Mo Lee, and Ho-Jung Kang.
Visualization: Young-Mi Kang, Su-Keon Lee, Yun-Rak Choi, and Seong-Hwan Moon.
Writing—original draft: Young-Mi Kang, Su-Keon Lee, and Yun-Rak Choi.
Writing—review & editing: Young-Mi Kang, Su-Keon Lee, Yun-Rak Choi, and Seong-Hwan Moon.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download