INTRODUCTION
MATERIALS AND METHODS
Patient characteristics
Laboratory assay and definitions
Serum AHRT activity assay
Mitochondria inhibiting activity assay
Statistical analysis
RESULTS
Clinical characteristics of the study patients
Table 1
Comparison of Baseline Characteristics of the Study Groups
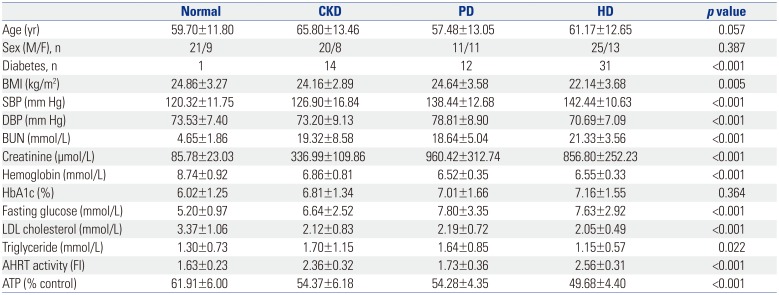
CKD, chronic kidney disease; PD, peritoneal dialysis; HD, hemodialysis; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; BUN, blood urea nitrogen; LDL, low-density-lipoprotein; AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; FI, fold induction. Values are expressed as means±SDs unless otherwise noticed. Significance at p<0.05 by ANOVA and Tukey's test or χ2-test.
Comparisons of AHRT activity and intracellular ATP levels according to kidney function and dialysis modalities
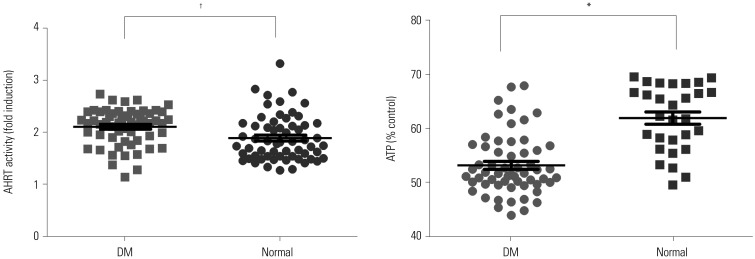
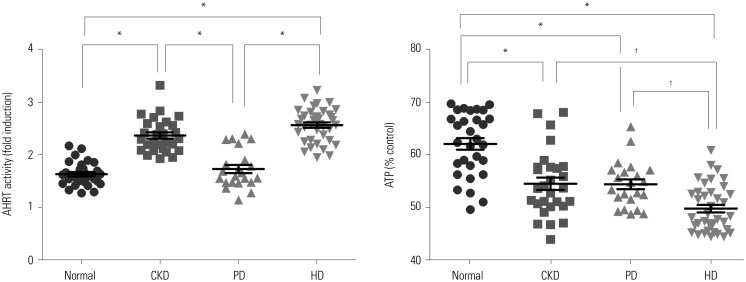 | Fig. 1Comparison of AHRT activity and intracellular ATP levels among study groups. Significance at p<0.05 by ANOVA and Tukey's test . *p<0.001, †p<0.05. AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; CKD, chronic kidney disease; PD, peritoneal dialysis; HD, hemodialysis. |
Comparison of AHRT activities and intracellular ATP levels according to diabetes status in all study participants
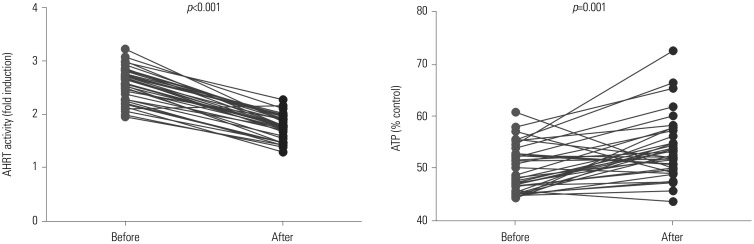
Comparison of AHRT activity and ATP levels according to dialysis treatment and dialysis adequacy
Correlation analysis for AHRT activity, intracellular ATP levels, and various clinical parameters
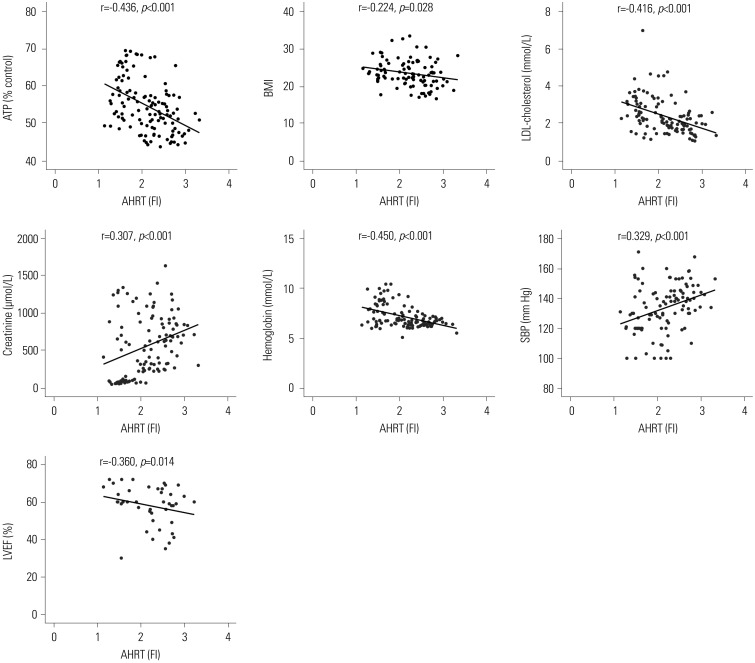 | Fig. 4Significant correlations among AHRT activity and various clinical parameters. Pearson's coefficient r and p values are presented. AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; BMI, body mass index; LDL, low-density-lipoprotein; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; FI, fold induction. |
Table 2
Correlations among AHRT Activity, Intracellular ATP, and Various Clinical Parameters
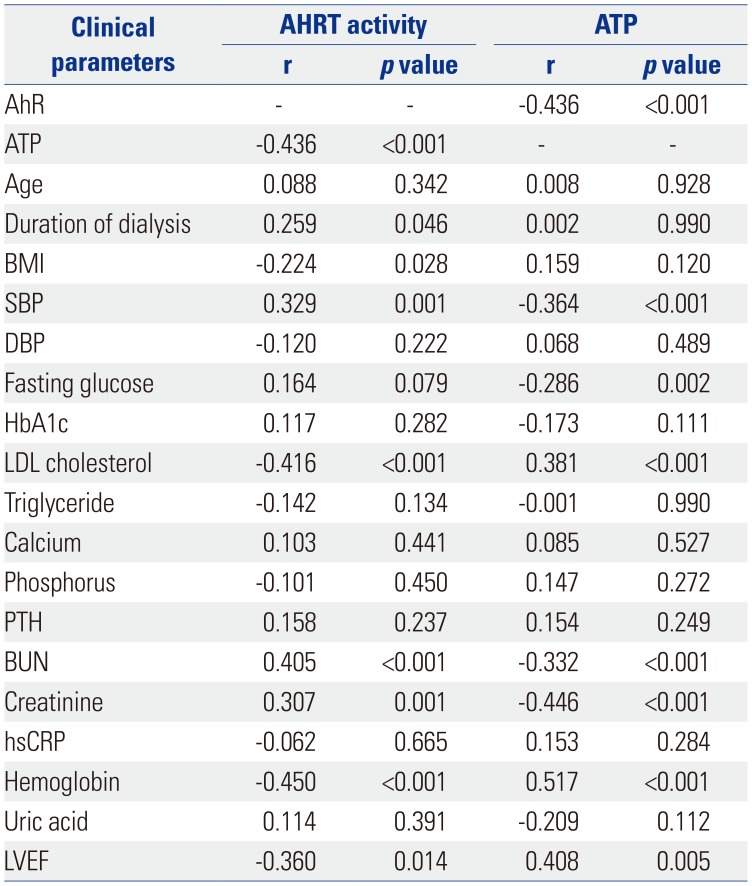
AhR, aryl hydrocarbon receptor; AHRT, AhR transactivating; ATP, adenosine triphosphate; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; LDL, low-density-lipoprotein; PTH, parathyroid hormone; BUN, blood urea nitrogen; hsCRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction.
Pearson's coefficient r and p values are presented.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download