1. Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009; 53:2288–2295. PMID:
19520254.

2. Borger MA, Preston M, Ivanov J, Fedak PW, Davierwala P, Armstrong S, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004; 128:677–683. PMID:
15514594.

3. Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002; 73:17–27. PMID:
11834007.

4. Vega J, Gonzalez D, Yankovic W, Oroz J, Guaman R, Castro N. Thoracic aortic aneurysm. Natural history, diagnosis and management. Rev Chil Cardiol. 2014; 33:127–135.
5. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014; 35:2873–2926. PMID:
25173340.
6. Lee SH, Kim JB, Kim DH, Jung SH, Choo SJ, Chung CH, et al. Management of dilated ascending aorta during aortic valve replacement: valve replacement alone versus aorta wrapping versus aorta replacement. J Thorac Cardiovasc Surg. 2013; 146:802–809. PMID:
23856198.
7. Norton E, Yang B. Managing thoracic aortic aneurysm in patients with bicuspid aortic valve based on aortic root-involvement. Front Physiol. 2017; 8:397. PMID:
28659818.

8. Yasuda H, Nakatani S, Stugaard M, Tsujita-Kuroda Y, Bando K, Kobayashi J, et al. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation. 2003; 108 Suppl 1:II291–II294. PMID:
12970248.

9. Della Corte A, Bancone C, Quarto C, Dialetto G, Covino FE, Scardone M, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007; 31:397–404. PMID:
17236783.

10. Ius F, Koigeldiyev N, Roumieh M, Ismail I, Tudorache I, Shrestha M, et al. Impact of sinuses of Valsalva on prosthesis durability in patients undergoing ascending aorta and aortic valve replacement with Carpentier-Edwards bioprosthesis: a propensity score-based study. Eur J Cardiothorac Surg. 2016; 49:1676–1684. PMID:
26656448.

11. Plonek T. A meta analysis and systematic review of wrapping of the ascending aorta. J Card Surg. 2014; 29:809–815. PMID:
25195510.
12. Belov IV, Stepanenko AB, Gens AP, Savichev DD, Charchyan ER. Reduction aortoplasty for ascending aortic aneurysm: a 14-year experience. Asian Cardiovasc Thorac Ann. 2009; 17:162–166. PMID:
19592547.

13. Ogus NT, Ciçek S, Isik O. Selective management of high risk patients with an ascending aortic dilatation during aortic valve replacement. J Cardiovasc Surg (Torino). 2002; 43:609–615.
14. González-Santos JM, Arnáiz-García ME. Wrapping of the ascending aorta revisited-is there any role left for conservative treatment of ascending aortic aneurysm? J Thorac Dis. 2017; 9(Suppl 6):S488–S497. PMID:
28616345.

15. Choi MS, Jeong DS, Lee HY, Sung K, Kim WS, Lee YT, et al. Aortic wrapping for a dilated ascending aorta in bicuspid aortic stenosis. Circ J. 2015; 79:778–784. PMID:
25740349.

16. Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM 3rd. Fate of nonreplaced sinuses of Valsalva in bicuspid aortic valve disease. J Thorac Cardiovasc Surg. 2011; 142:278–284. PMID:
21036368.

17. Zhang H, Lu F, Qu D, Han L, Xu J, Ji G, et al. Treatment of fusiform ascending aortic aneurysms: a comparative study with 2 options. J Thorac Cardiovasc Surg. 2011; 141:738–743. PMID:
20584536.

18. Xuan Y, Wang Z, Liu R, Haraldsson H, Hope MD, Saloner DA, et al. Wall stress on ascending thoracic aortic aneurysms with bicuspid compared with tricuspid aortic valve. J Thorac Cardiovasc Surg. 2018; 156:492–500. PMID:
29656820.

19. Randall RD Jr, Walley BD, Meredith JH. Comparison of polytetrafluoroethylene (PTFE) and dacron as long, small-diameter arterial grafts in dogs. Am Surg. 1982; 48:622–627. PMID:
6218768.
20. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease Representative Members. Hiratzka LF, Creager MA, Isselbacher EM, Svensson LG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease Representative Members. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016; 133:680–686. PMID:
26637530.

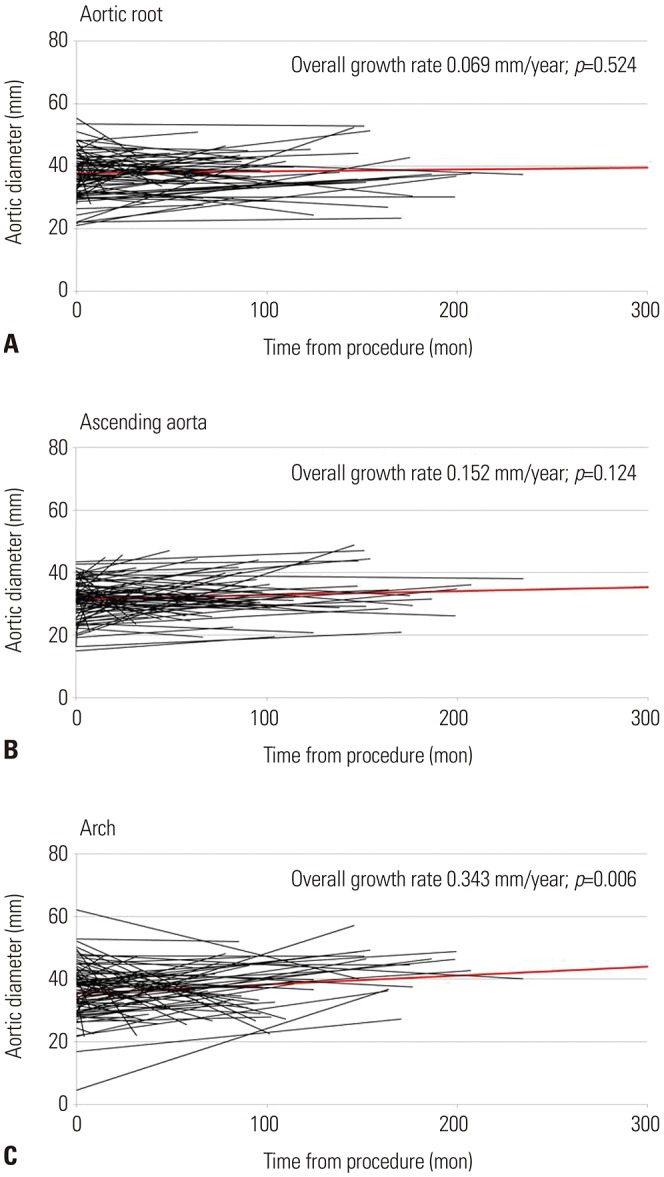
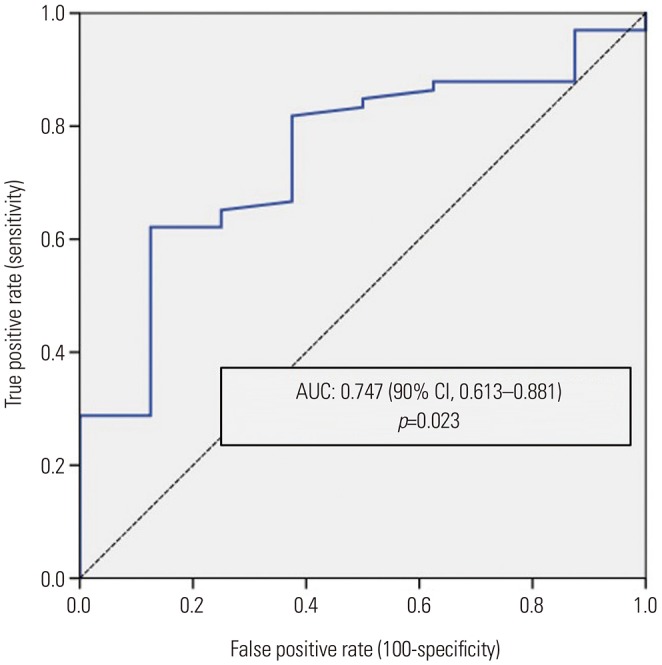

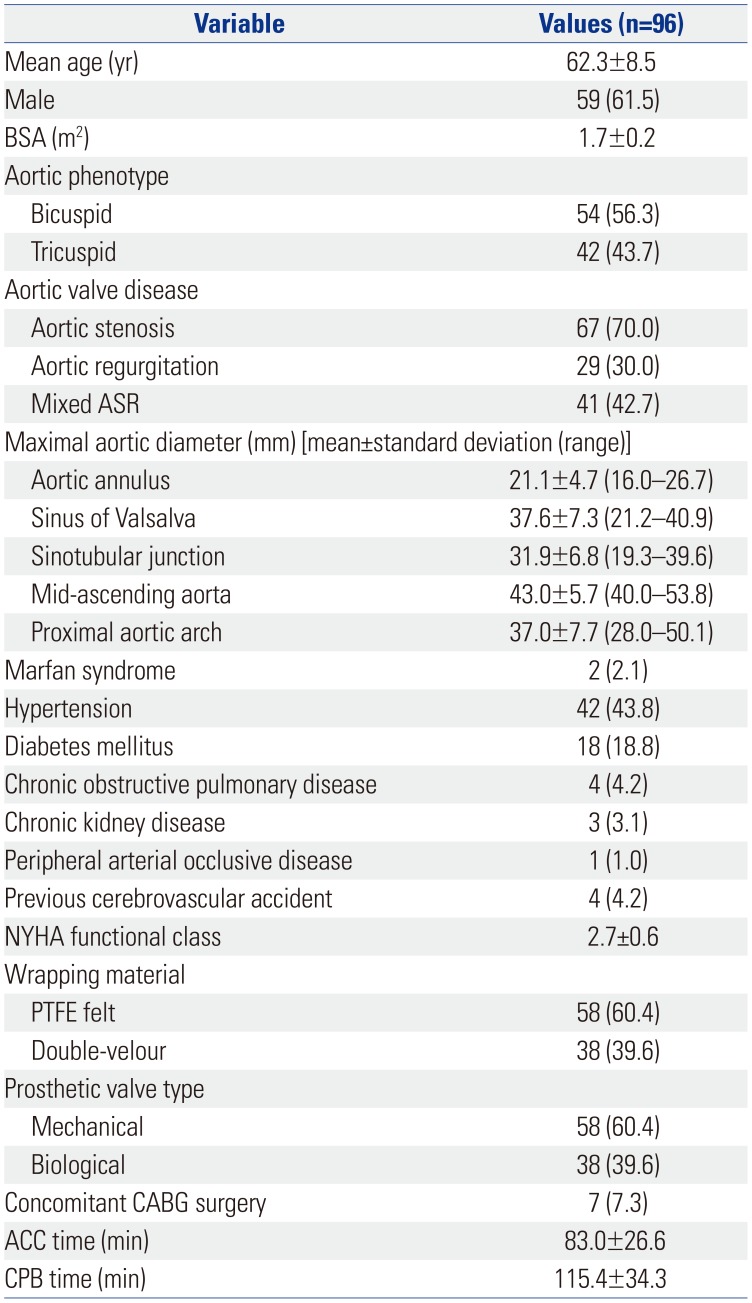
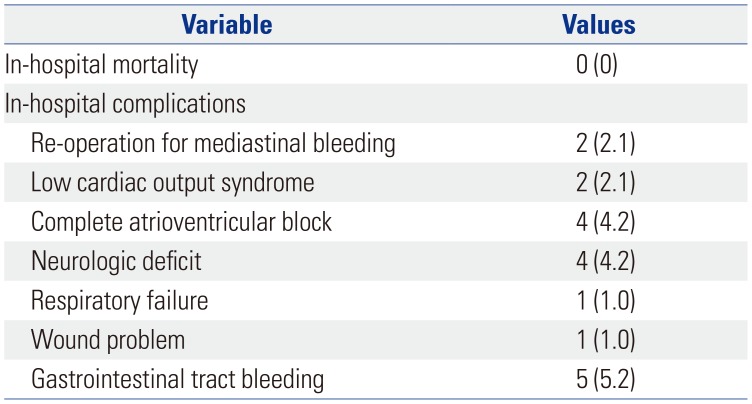




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download