Abstract
Purpose
Materials and Methods
Results
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: Geu-Ru Hong, Sang Jin Ha.
Data curation: Sang Jin Ha.
Formal analysis: Sang Jin Ha.
Funding acquisition: Geu-Ru Hong.
Investigation: Sang Jin Ha, Geu-Ru Hong, Myeong-Ki Hong.
Methodology: Sang Jin Ha, Geu-Ru Hong.
Project administration: Geu-Ru Hong.
Resources: Myeong-Ki Hong, Geu-Ru Hong.
Software: Sang Jin Ha, Geu-Ru Hong.
Supervision: Geu-Ru Hong, Myeong-Ki Hong.
Validation: Sang Jin Ha, Sang-Yong Yoo, Geu-Ru Hong, Myeong-Ki Hong.
Visualization: Sang Jin Ha.
Writing—original draft: Sang Jin Ha, Geu-Ru Hong.
Writing—review & editing: Sang Jin Ha, Sang-Yong Yoo, Geu-Ru Hong, Myeong-Ki Hong.
References
Fig. 1
Hemodynamic changes over time. (A) Serial change of aortic valve peak velocity. (B) Serial change of MSPG across the aortic valve. Error bars represent standard deviations, while the error bar of the left ventricular mass index represents the standard error. TAVR: transcatheter aortic valve replacement; sAVR: surgical aortic valve replacement; AVmax: peak AV velocity; MSPG: mean systolic pressure gradients; Pre: before the procedure; mo: months.
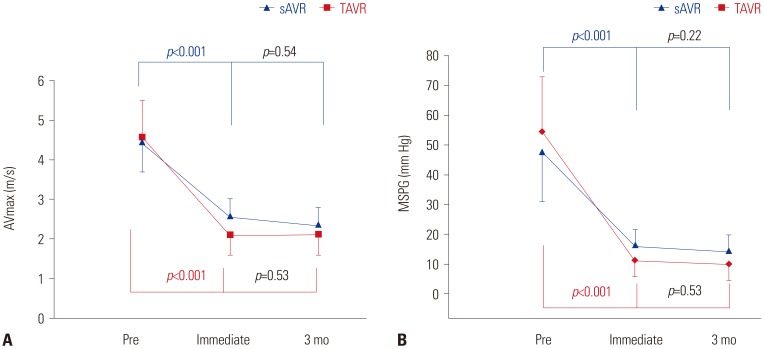
Fig. 2
Distribution of severity of diastolic function changes in TAVR and sAVR groups over time. Comparison between TAVR and sAVR at immediately after the procedure, p=0.018. Comparison between TAVR and sAVR at 3 months later, p=0.88. TAVR, transcatheter aortic valve replacement; sAVR, surgical aortic valve replacement; Pre, before the procedure.
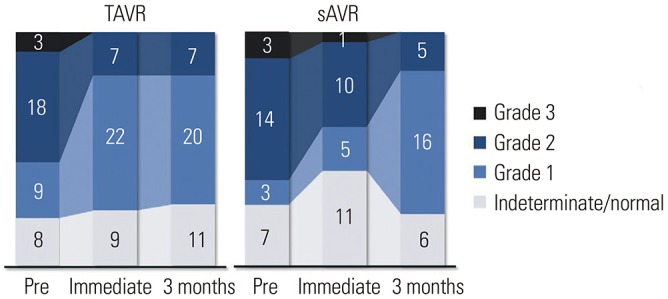
Fig. 3
Changes in diastolic function parameters over time. (A) Serial change of E velocity. (B) Serial change of e′ velocity. (C) Serial change of E/e′ ratio. (D) Serial change of RVSP. The error bars represent one standard deviation. E/e′: E velocity to e′ ratio; RVSP: right ventricular systolic pressure; TAVR: transcatheter aortic valve replacement; sAVR: surgical aortic valve replacement; Pre: before the procedure; mo: months.
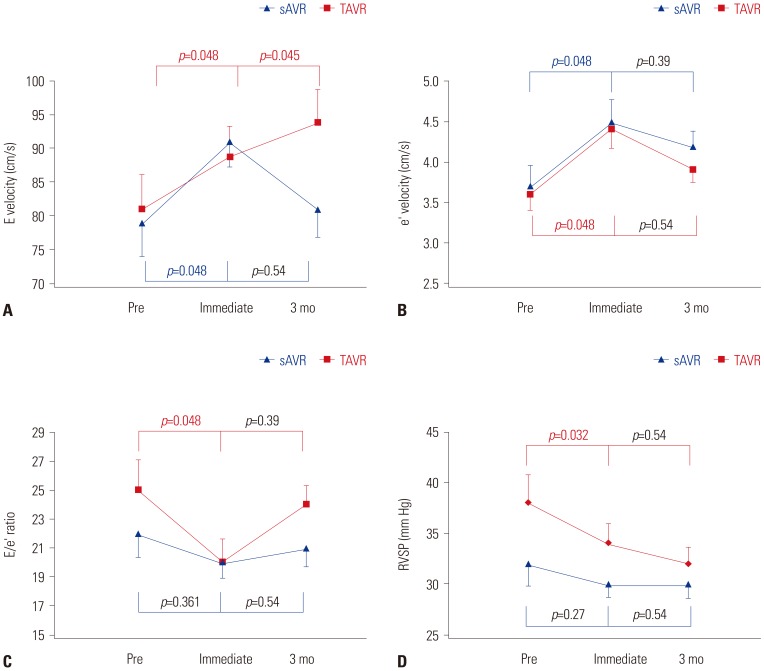
Fig. 4
Changes in afterload parameters over time. (A) Serial change of valvuloarterial impedance. (B) Serial change of SAC. (C) Serial change of SVR. Error bars represent one standard error. TAVR: transcatheter aortic valve replacement; sAVR: surgical aortic valve replacement; Zva: valvuloarterial impedance; SAC: systemic arterial compliance; SVR: systemic vascular resistance; Pre: before the procedure; mo: months.
Table 1
Baseline Characteristics of the TAVR and sAVR Groups
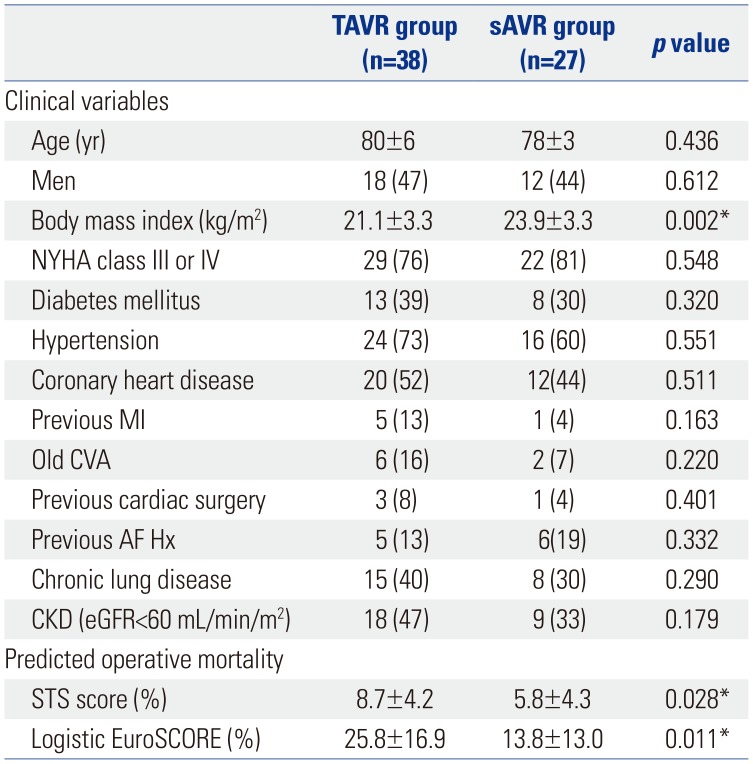
TAVR, transcatheter aortic valve replacement; sAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; CVA, cerebrovascular accident; MI, myocardial infarction; PCI, percutaneous coronary intervention; OP, operation; Hx, history; AF, atrial fibrillation; CKD, chronic kidney disease; NYHA, New York Heart Association; eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Operative Risk Evaluation score.
Data are presented as means±standard deviations or n (%).
*p<0.05.
Table 2
Baseline Echocardiographic Findings of the TAVR and sAVR Groups
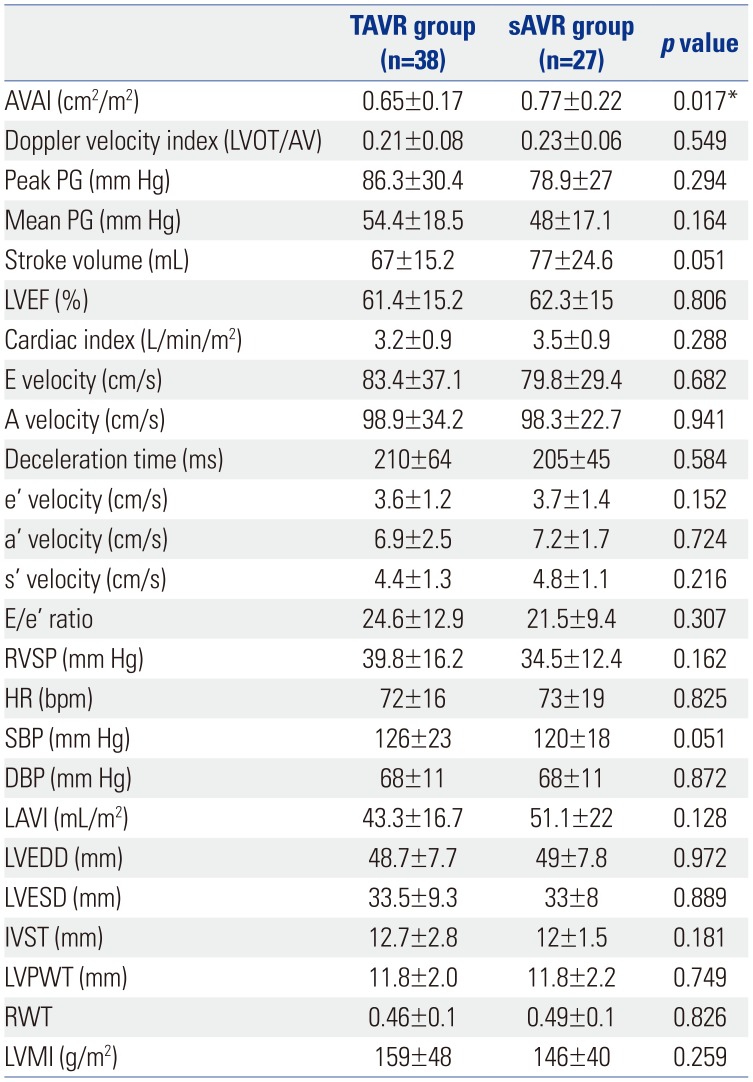
TAVR, transcatheter aortic valve replacement; sAVR, surgical aortic valve replacement; LVOT, left ventricular outflow tract; AVAI, aortic valve area index; PG, pressure gradient; LVEF, left ventricular ejection fraction; E/e′, E wave to e′ ratio; RVSP, right ventricular systolic pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LAVI, left ventricular volume index; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVST, interventricular septal thickness; LVPWT, left ventricular posterior wall thickness; RWT, relative wall thickness; LVMI, left ventricular mass index.
Data are presented as means±standard deviations.
*p<0.05.
Table 3
Hemodynamic Improvement after TAVR and sAVR
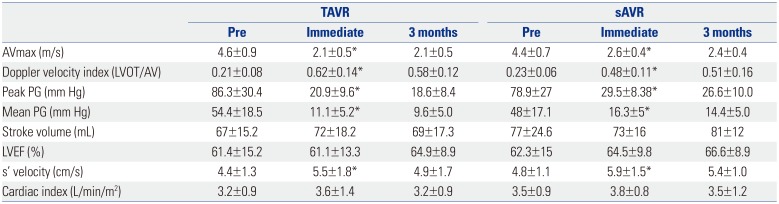
TAVR, transcatheter aortic valve replacement; sAVR, surgical aortic valve replacement; LVOT, left ventricular outflow tract; AVmax, peak aortic valve velocity; PG, pressure gradient; LVEF, left ventricular ejection fraction.
Data are presented as means±standard deviations.
*Effect between pre-procedure and immediate procedure: p<0.05
Table 4
Changes in Diastolic Function over Time
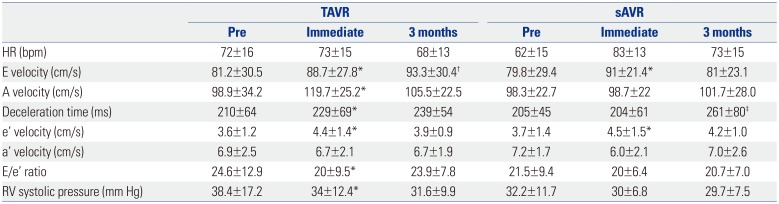
TAVR, transcatheter aortic valve replacement; sAVR, surgical aortic valve replacement; HR, heart rate; E/e′, E wave to e′ ratio; RV, right ventricle.
Data are presented as means±standard deviations.
*Effect between pre-procedure and immediately after procedure: p<0.05, †Effect between immediate procedure and after 3 months: p<0.05, ‡Significant difference only after 3 months: p<0.05
Table 5
LA and LV Structural Changes over Time
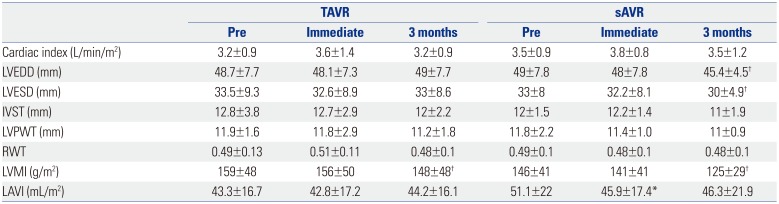
TAVR, transcatheter aortic valve replacement; sAVR, surgical aortic valve replacement; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVST, interventricular septal thickness; LVPWT, left ventricular posterior wall thickness; RWT, relative wall thickness; LVMI, left ventricular mass index; LAVI, left atrial volume index.
Data are presented as means±SDs.
*Effect between pre-procedure and immediate procedure: p<0.05, †Significant difference only after 3 months: p<0.05




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download