Abstract
Purpose
The purpose of this study was to assess the diagnostic and prognostic value of serum progastrin-releasing peptide (ProGRP) in patients with gastric cancer (GC).
Materials and Methods
A total of 150 patients with GC (89 males and 61 females) were recruited, including those with stage I (n=28), stage II (n=33), stage III (n=50), and stage IV (n=39) disease; 50 healthy controls and 66 patients with benign gastric diseases were also enrolled. Levels of serum ProGRP, carcinoembryonic antigen (CEA), and carbohydrate antigen 72-4 (CA72-4) were measured in all subjects.
Results
Serum ProGRP levels were significantly higher in GC patients than in controls (p<0.001), and ProGRP was significantly correlated with tumor size, tumor node metastasis stage, differentiation, invasion depth, and lymph node metastasis (p< 0.005). ProGRP levels were significantly decreased after chemotherapy (p<0.001). Receiver operating characteristic curves revealed a sensitivity and specificity for serum ProGRP in GC of 85.9% and 81.2%, respectively. ProGRP levels were positively correlated with CA72-4 and CEA (r=0.792 and 0.688, p<0.05, respectively). Combined detection of ProGRP, CEA, and CA72-4 showed the best diagnostic power for GC.
Gastric cancer (GC) is one of the most prevalent cancers worldwide and has an especially poor prognosis.1 Despite new chemotherapeutic regimens and improved surgical outcomes, GC remains one of the three leading causes of cancer-related death worldwide.2 Depth of invasion and the presence of lymph node metastases are considered to be the most important prognostic factors in GC.345 Nevertheless, more than 80% of patients are diagnosed at a moderate or advanced stage, which usually delays the best treatment opportunity.6 Although gastroscopy is a reasonable method for diagnosing GC, it is not suitable for general investigation in patients with subclinical symptoms. Therefore, novel biomarkers that can both predict the diagnosis and prognosis of and guide treatments for patients with GC are desired.
Gastrin-releasing peptide (GRP) is a neuropeptide hormone that was originally isolated from porcine gastric tissue.7 It is widely distributed throughout the mammalian nervous system, as well as the gastrointestinal and pulmonary tracts.8910 Progastrin-releasing peptide (ProGRP) is a precursor form and a more stable precursor of GRP, which is a biologically active protein that stimulates tumor cell proliferation. It appears that the growth-stimulating properties of ProGRP may be responsible for aggressive tumor behavior. To date, numerous studies have demonstrated that ProGRP is a biomarker of small cell lung cancer.111213 However, few studies have measured ProGRP levels in patients with GC and prospectively evaluated associations between ProGRP levels and diagnosis or treatment of GC. The objectives of this study were to analyze serum ProGRP levels in GC patients, to investigate associations among serum ProGRP levels and clinicopathological parameters, and to evaluate the diagnostic and prognostic value of serum ProGRP for GC.
A total of 150 patients with GC were recruited from Binhai County People's Hospital from January 2014 to December 2017. This group was composed of 89 males and 61 females, with a mean age of 57.2±10.6 years (range 33–79 years). GC tumor node metastasis (TNM) staging was determined based on the 7th American Joint Committee on Cancer (AJCC) TNM Staging Classification for Carcinoma of the Stomach.14 According to AJCC staging, 28 patients had stage I, 33 patients had stage II, 50 patients had stage III, and 39 patients had stage IV disease. The cases of GC were classified according to histologic characteristics into 47 papillary adenocarcinomas, 55 tubular adenocarcinomas, 23 mucoid carcinomas, and 25 signet ring cell carcinomas. In addition, 66 patients diagnosed with benign gastric disease at the Gastroenterology Endoscopy Center and 50 healthy subjects examined at the Health Examination Center over the same study period were enrolled. The 66 patients with benign gastric disease included 40 males and 26 females, with a mean age of 55.4±13.1 years (range 30–68 years). The benign gastric diseases included 20 cases of superficial gastritis, 17 cases of atrophic gastritis, 13 cases of peptic ulcer disease, and 16 cases of gastric polyps. The 50 healthy subjects included 28 males and 22 females, with a mean age of 40.2±10.8 years (range 24–69 years). There were no differences in baseline data, such as age, sex, etc., among the three groups (p>0.05). None of the healthy individuals had a personal/family history of GC, and patients with neuroendocrine tumors or renal failure were excluded. The study was approved by the Human Investigation Committee of Binhai County People's Hospital (BHEA, 2014-10), and written informed consent was obtained from all study participants. The study was carried out in accordance with the guidelines of the Declaration of Helsinki.
Blood samples were collected from the patients in the morning between 7:00 and 8:00 a.m. before chemotherapy and after chemotherapy in three cycles. Blood samples were obtained by venous puncture and centrifuged at 2000 ×g for 10 min. The serum was stored at −80℃ until ready for analysis. An automatic chemiluminescence immune analyzer with respective kits (Abbott Japan, Tokyo, Japan) was used to determine the levels of serum ProGRP, carcinoembryonic antigen (CEA), and carbohydrate antigen 72-4 (CA72-4) in strict accordance with the kit's instructions. Serum levels of ProGRP were analyzed using ARCHITECT i2000 (Abbott, Chicago, IL, USA), and serum levels of CEA and CA72-4 were measured with Cobas E601 (Roche, Mannheim, Germany).
All analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as mean±standard deviation. Comparisons of means among groups were analyzed by the Kruskal-Wallis test, Mann-Whitney U, and Student t-test. The χ2 test and Fisher exact test were used to assess the significance of differences in diagnostic power for GC between detection of ProGRP, CEA, and CA72-4 combined and individually. Correlations within each group were evaluated using Spearman's or Pearson's method. Receiver operating characteristic (ROC) curves were constructed to determine the sensitivity and specificity of serum ProGRP for discriminating GC patients at optimal cut-off points. p<0.05 was considered statistically significant.
Comparisons of serum ProGRP levels according to clinicopathological factors are shown in Table 1. Serum ProGRP levels in GC were significantly correlated with tumor size (p=0.014), TNM stage (p=0.031), differentiation (p=0.019), invasion depth (p=0.028), and lymph node metastasis (p=0.018). However, serum ProGRP levels showed no associations with sex (p=0.213), age (p=0.086), or tumor location (p=0.258). These results indicated that serum ProGRP is related to GC metastasis (Table 1). Serum ProGRP levels did not differ significantly according to histopathological characteristics in patients with GC (p>0.05) (Table 2).
The serum levels of ProGRP were 249.3±28.9 pg/mL in patients with GC, 20.1±5.9 pg/mL in patients with gastric benign disease, and 14.4±7.6 pg/mL in healthy controls. Serum ProGRP levels in patients with GC were significantly higher than those in patients with benign disease and healthy controls (p<0.001), whereas no difference therein was found between benign disease patients and healthy controls (p=0.185) (Fig. 1).
In total, 108 patients with advanced GC were treated with chemotherapy based on fluoropymidine plus cisplatin regimens in three cycles. Analyzing changes in ProGRP levels before and after chemotherapy, we found ProGRP levels to be significantly decreased after chemotherapy (249.3±28.9 pg/mL vs. 26.1±12.8 pg/mL, p<0.001).
The diagnostic performance of serum ProGRP was evaluated using ROC analysis. The area under the curve value for serum ProGRP was 0.713 (95% CI: 0.631–0.795). The cut-off value for ProGRP, as calculated by ROC, was 28.3 pg/mL. The sensitivity and specificity of serum ProGRP for GC were 85.9% and 81.2%, respectively (Fig. 2).
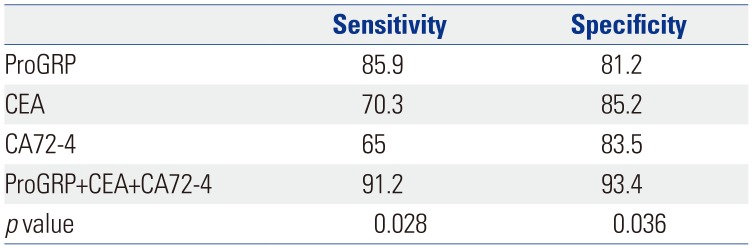
Combining multiple markers rather than relying on a single marker can improve both sensitivity and specificity. The sensitivity and specificity of combined detection of ProGRP, CEA, and CA72-4 were 91.2% and 93.4%, respectively, which was better than those for single detections (p<0.05), as shown in Table 3.
Serum ProGRP levels showed positive correlations with CEA and CA72-4 in patients with GC (r=0.680 and 0.773, respectively, p<0.01, Fig. 3).
Although applications of imaging and endoscopic examinations play important roles in the diagnosis of GC,1516 examination therewith are complex and expensive. Detection of tumor markers is convenient and rapid, and may reflect the occurrence and development of tumors in a timely manner.17 ProGRP has been verified as a biomarker for neuroendocrine origin. It acts via ProGRP receptor and may be involved in a multitude of physiological functions in certain cancers.18 In the present study, we observed that ProGRP levels in patients with GC are significantly increased, compared to those in patients with gastric benign disease and in healthy controls (p<0.001). Several studies have shown that ProGRP is rarely elevated in benign conditions, except in patients with renal failure.192021222324 Moreover, Molina, et al.10 reported that only a small proportion of patients with benign disease have a ProGRP level above 50 pg/mL. We obtained similar results: although ProGRP levels in patients with gastric benign disease were slightly higher than those in healthy controls, the difference was not statistically significant (p>0.05). Our results indicate that ProGRP may be tumor marker for GC.
The present study revealed that ProGRP levels in GC are closely correlated with tumor size, TNM stage, differentiation, invasion depth, and lymph node metastasis (p<0.005). There were no differences according to histopathological results (p>0.005). At a cut-off level of 28.3 pg/mL, serum ProGRP had a sensitivity of 85.9% and a specificity of 81.2% for predicting GC. Accordingly, we suggest that ProGRP may be useful as a potential prognostic factor for GC, though its role in GC progression requires further investigation.
To explore the important role of ProGRP in chemotherapy of GC, we analyzed serum ProGRP levels during chemotherapy treatment. The results revealed that serum ProGRP levels significantly decreased after chemotherapy, with levels being significantly different before and after chemotherapy (p<0.001). Our findings indicated that ProGRP may be useful in monitoring responses to therapy.
CEA is a cancer embryo antigen located on chromosome 19, and it is commonly used for the diagnosis of malignant tumors of the digestive tract. Meanwhile, CA72-4 is a high molecular weight glycoprotein antigen that is highly specific for GC and that has good specificity for GC.2526 Several studies have confirmed the sensitivity of tumor markers, either alone or in combination, for GC.272829 The present study explored the sensitivities and specificities of serum ProGRP, CEA, and CA72-4 as diagnostic makers for GC, and these improved to 91.2% and 93.4%, respectively, with the combined use of all three. Moreover, we confirmed positive correlations between ProGRP and CA72-4 and CEA in patients with GC. Accordingly, we suggest that the combined detection of ProGRP, CEA, and CA72-4 has good diagnostic power for GC.
The limitations of this study should be mentioned. The sample size of the present cross-sectional study was relatively small. In particular, there were too few patients who were followed for a long enough time to ascertain the significance of the biomarkers in relation to responses to chemotherapy. In addition, there was no mechanistic investigation in this study, and further studies on interactions of ProGRP in GC are necessary.
In summary, this study presents evidence that serum ProGRP levels are significantly elevated in GC patients and that serum ProGRP levels are significantly correlated with lymph node metastasis and distant metastasis. ProGRP levels were significantly decreased after chemotherapy. Showing moderate sensitivity and specificity in GC diagnosis, ProGRP might be useful as a biomarker for GC diagnosis and could be a potential biomarker in prediction of chemotherapy efficacy.
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: Xiaodong Yin.
Data curation: Li Li.
Formal analysis: Hai Meng.
Funding acquisition: Juanyu Hu.
Investigation: Zhengqing Yu.
Methodology: Jianyong Xu.
Project administration: Li Li.
Resources: Xiaodong Yin and Hai Meng.
Software: Juanyu Hu.
Supervision: Li Li.
Validation: Xiaodong Yin and Hai Meng.
Visualization: Hai Meng and Juanyu Hu.
Writing—original draft: Xiaodong Yin and Jianyong Xu.
Writing—review & editing: Li Li and Xiaodong Yin.
References
1. Wang Y, Wang M. Prognostic significance of expression of cysteinerich 61 and cyclooxygenase-2 in gastric cancer. BMC Gastroenterol. 2016; 16:74. PMID: 27457107.

2. Seol SY, Kim C, Lim JY, Yoon SO, Hong SW, Kim JW, et al. Overexpression of endoplasmic reticulum oxidoreductin 1-α (ERO1L) is associated with poor prognosis of gastric cancer. Cancer Res Treat. 2016; 48:1196–1209. PMID: 26987398.

3. Qu Y, Zhou C, Zhang J, Cai Q, Li J, Du T, et al. The metastasis suppressor SOX11 is an independent prognostic factor for improved survival in gastric cancer. Int J Oncol. 2014; 44:1512–1520. PMID: 24604109.

4. Jo MJ, Lee JH, Nam BH, Kook MC, Ryu KW, Choi IJ, et al. Preoperative serum angiopoietin-2 levels correlate with lymph node status in patients with early gastric cancer. Ann Surg Oncol. 2009; 16:2052–2057. PMID: 19408052.

5. Jin C, Lin JR, Ma L, Song Y, Shi YX, Jiang P, et al. Elevated spondin-2 expression correlates with progression and prognosis in gastric cancer. Oncotarget. 2017; 8:10416–10424. PMID: 28060752.

6. Mayer RJ, Venook AP, Schilsky RL. Progress against GI cancer during the American Society of Clinical Oncology’s first 50 years. J Clin Oncol. 2014; 32:1521–1530. PMID: 24752046.

7. Taira N, Kawabata T, Ichi T, Kushi K, Yohena T, Kawasaki H, et al. Utility of the serum ProGRP level for follow-up of pulmonary carcinoid tumors. Am J Case Rep. 2014; 15:337–339. PMID: 25109370.

8. Ischia J, Patel O, Bolton D, Shulkes A, Baldwin GS. Expression and function of gastrin-releasing peptide (GRP) in normal and cancerous urological tissues. BJU Int. 2014; 113 Suppl 2:40–47. PMID: 24894852.

9. Lv SP, Wang Y, Huang L, Wang F, Zhou JG, Ma H. Meta-analysis of serum gastrin-releasing peptide precursor as a biomarker for diagnosis of small cell lung cancer. Asian Pac J Cancer Prev. 2017; 18:391–397. PMID: 28345820.
10. Molina R, Filella X, Augé JM. ProGRP: a new biomarker for small cell lung cancer. Clin Biochem. 2004; 37:505–511. PMID: 15234231.

11. Oh HJ, Park HY, Kim KH, Park CK, Shin HJ, Lim JH, et al. Progastrin-releasing peptide as a diagnostic and therapeutic biomarker of small cell lung cancer. J Thorac Dis. 2016; 8:2530–2537. PMID: 27747005.

12. Molina R, Auge JM, Filella X, Viñolas N, Alicarte J, Domingo JM, et al. Pro-gastrin-releasing peptide (ProGRP) in patients with benign and malignant diseases: comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 2005; 25(3A):1773–1778. PMID: 16033098.
13. Korse CM, Holdenrieder S, Zhi XY, Zhang X, Qiu L, Geistanger A, et al. Multicenter evaluation of a new progastrin-releasing peptide (ProGRP) immunoassay across Europe and China. Clin Chim Acta. 2015; 438:388–395. PMID: 25262909.

14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.

15. Pan Z, Pang L, Ding B, Yan C, Zhang H, Du L, et al. Gastric cancer staging with dual energy spectral CT imaging. PLoS One. 2013; 8:e53651. PMID: 23424614.

16. Chen XJ, Li N, Huang YD, Ren S, Liu F, Chen L, et al. Factors for postoperative gallstone occurrence in patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014; 15:877–881. PMID: 24568511.

17. Durães C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014; 464:367–378. PMID: 24487788.

18. Gong Z, Lu R, Xie S, Jiang M, Liu K, Xiao R, et al. Overexpression of pro-gastrin releasing peptide promotes the cell proliferation and progression in small cell lung cancer. Biochem Biophys Res Commun. 2016; 479:312–318. PMID: 27639644.

19. Molina R, Augé JM, Bosch X, Escudero JM, Viñolas N, Marrades R, et al. Usefulness of serum tumor markers, including progastrin-releasing peptide in patients with lung cancer: correlation with histology. Tumour Biol. 2009; 30:121–129. PMID: 19506400.

20. Nakahama H, Tanaka Y, Fujita Y, Fujii M, Sugita M. CYFRA 21-1 and ProGRP, tumor markers of lung cancer, are elevated in chronic renal failure patients. Respirology. 1998; 3:207–210. PMID: 9767622.

21. Inaji H, Komoike Y, Motomura K, Higashiyama M, Ohtsuru M, Funai H, et al. Demonstration and diagnostic significance of pro-gastrin-releasing peptide in medullary thyroid carcinoma. Oncology. 2000; 59:122–125. PMID: 10971170.

22. Molina R, Auge JM, Alicarte J, Filella X, Viñolas N, Ballesta AM. Pro-gastrin-releasing peptide in patients with benign and malignant disease. Tumour Biol. 2004; 25:56–61. PMID: 15192313.
23. Molina R, Bosch X, Auge JM, Filella X, Escudero JM, Molina V, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012; 33:463–474. PMID: 22161237.

24. Korse CM, Taal BG, Bonfrer JM, Vincent A, van Velthuysen ML, Baas P. An elevated progastrin-releasing peptide level in patients with well-differentiated neuroendocrine tumours indicates a primary tumour in the lung and predicts a shorter survival. Ann Oncol. 2011; 22:2625–2630. PMID: 21415235.

25. Yin LK, Sun XQ, Mou DZ. Value of combined detection of serum CEA, CA72-4, CA19-9 and TSGF in the diagnosis of gastric cancer. Asian Pac J Cancer Prev. 2015; 16:3867–3870. PMID: 25987051.

26. Yu J, Zhang S, Zhao B. Differences and correlation of serum CEA, CA19-9 and CA72-4 in gastric cancer. Mol Clin Oncol. 2016; 4:441–449. PMID: 26998301.

27. Tian SB, Yu JC, Kang WM, Ma ZQ, Ye X, Cao ZJ, et al. Combined detection of CEA, CA 19-9, CA 242 and CA 50 in the diagnosis and prognosis of resectable gastric cancer. Asian Pac J Cancer Prev. 2014; 15:6295–6300. PMID: 25124614.

28. Liang Y, Wang W, Fang C, Raj SS, Hu WM, Li QW, et al. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016; 7:49565–49573. PMID: 27385101.

29. Yang AP, Liu J, Lei HY, Zhang QW, Zhao L, Yang GH. CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin Chim Acta. 2014; 437:183–186. PMID: 25086284.

Fig. 1
Comparison of serum progastrin-releasing peptide (ProGRP) levels in patients with gastric cancer (GC), patients with benign gastric disease, and healthy controls.
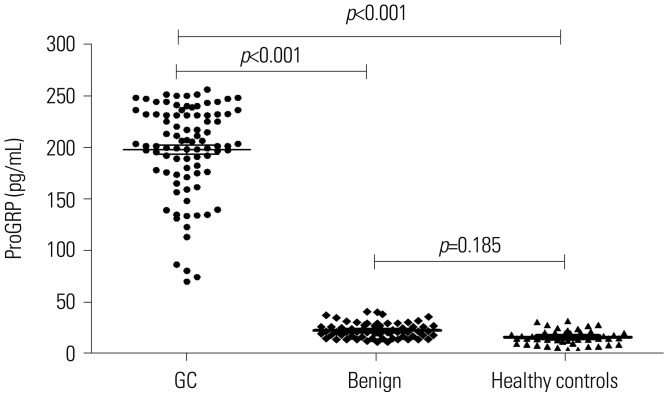
Fig. 2
Receiver operating characteristic (ROC) curve for serum progastrin-releasing peptide levels in patients with gastric cancer.
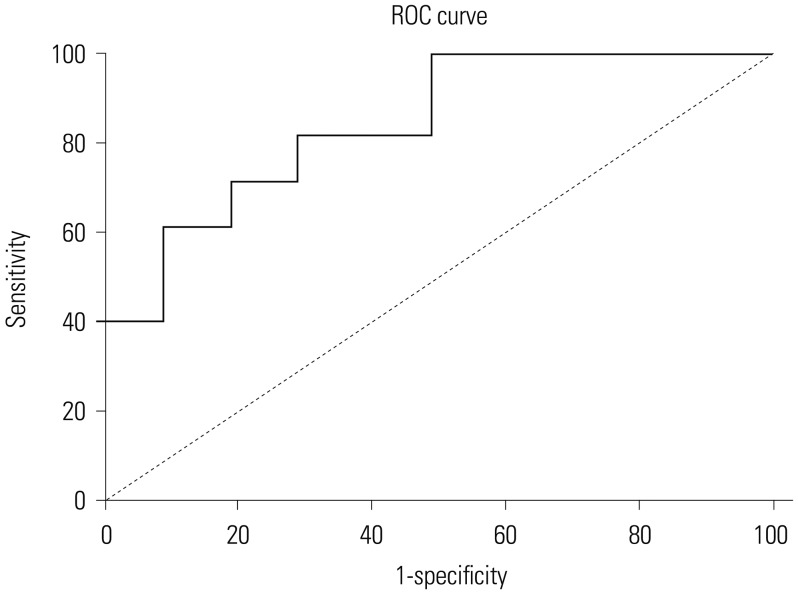
Fig. 3
Serum progastrin-releasing peptide (ProGRP) levels positively correlated with carcinoembryonic antigen (CEA) (A) and carbohydrate antigen 72-4 (CA72-4) (B), respectively, in patients with gastric cancer.
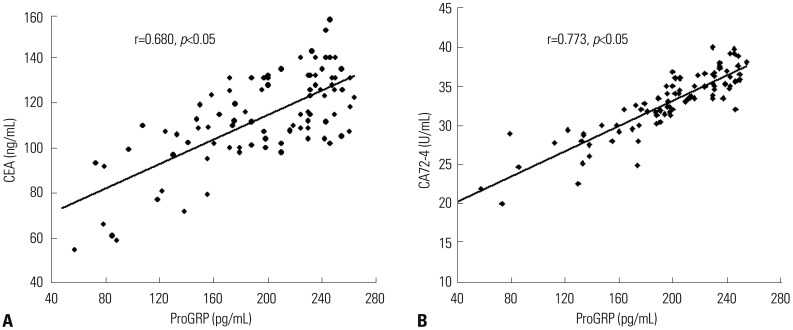
Table 1
Serum ProGRP Levels in Gastric Cancer Patients according to Clinicopathological Characteristics
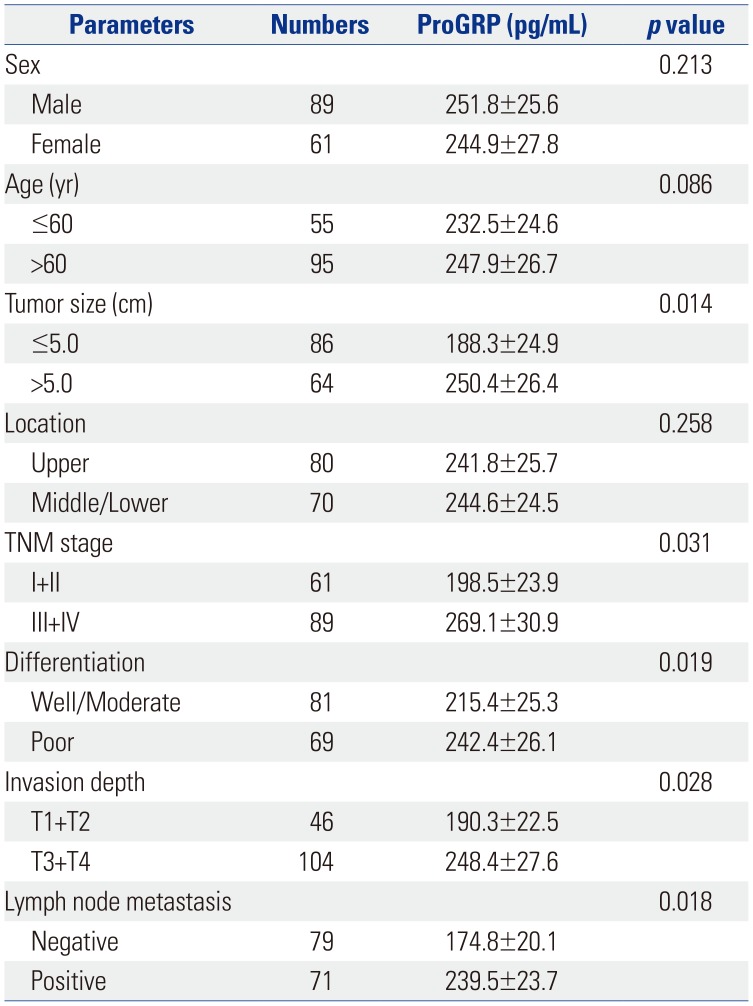
Table 2
Serum ProGRP Levels in Gastric Cancer Patients according to Histopathological Characteristics

| Papillary adenocarcinoma (n=47) | Tubular adenocarcinomas (n=55) | Mucoid carcinoma (n=23) | Signet ring cell carcinomas (n=25) | |
|---|---|---|---|---|
| ProGRP (pg/mL) | 248.3±27.8 | 243.3±25.1 | 255.3±33.6 | 253.3±35.2 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download