INTRODUCTION
METHODS
Animal model
Study protocol
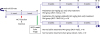 | Figure 1A schematic illustration of the preliminary experimental protocol is shown. Blue arrows indicate the time points at which body weight, echocardiography, and exercise capacity were assessed. The asterisk indicates the time point of the invasive pressure-volume hemodynamics assessment.MCT = monocrotaline; MAC = macitentan; N/S = normal saline; PAH = pulmonary arterial hypertension; SIL = sildenafil.
|
Drug administration
Physiological studies
Echocardiography
Exercise test and blood pressure monitoring
Invasive hemodynamic assessment
Histopathological analysis
Biochemical analysis of serum
Immunohistochemical analysis
Statistical analysis
RESULTS
Survival and changes in body weight
 | Figure 2(A) Kaplan-Meier survival curves show a clear difference in survival between the MCT group and the MAC-based treatment groups (MAC and MAC+SIL groups) (p=0.04). (B) Serial changes in body weight over 7 weeks of MCT injection are shown. (C) Changes in systolic and diastolic blood pressure are compared among groups.MAC = macitentan; MCT = monocrotaline; SIL = sildenafil.
*p<0.05 vs. the sham group; †p<0.05 vs. the MCT group; ‡p=0.07 vs. the sham group.
|
Hemodynamic study
Heart rate and systemic blood pressure
Exercise capacity
 | Figure 3(A) Comparisons in exercise capacity. Exercise capacity began to deteriorate one week after MCT injection in the MCT group, but not in the sham group. In contrast, exercise duration was relatively preserved in the MAC group (p<0.05). Exercise capacity in the MAC+SIL group was comparable to that in the MAC group (p>0.05). (B) Serial assessments of RV-FAC. Serial echocardiography showed that rats with MCT-induced PAH rats had reduced RV-FAC beginning at 5 weeks. Treatment with MAC alone or combination with MAC and SIL attenuated the progression of RV systolic dysfunction. (C) Serial assessments of the RV-EDD on echocardiography. (D) Serial assessments of the RV wall thickness. (E) PAAT was measured from the time of the onset of systolic flow to the time of the peak pulmonary outflow velocity. Note the significant differences in the PAAT among the 4 groups beginning at 3rd weeks. (F) Serial assessments of the TAPSE. (G) Serial assessment of RV-RA PG. TR jet could be reliably quantified via continuous-wave Doppler around 3 weeks after MCT injection in the MAC and MAC+SIL groups. TR tracing was difficult to follow in the sham group, and thus, the RV-RA PG could not be reliably obtained in the sham group. (H) Serial assessment of LV-EDD. LV-EDD was smaller in the MCT group, where D-shaped LV at the parasternal short axis image was clearly visualized, indicating development of severe pulmonary hypertension. In contrast, both the MAC and the MAC+SIL groups showed no significant decrement in LV dimension with preservation of the LV geometry.LV = left ventricular; LV-EDD = left ventricular end-diastolic dimensions; MCT = monocrotaline; MAC = macitentan; PAH = pulmonary arterial hypertension; PAAT = pulmonary artery acceleration time; RA = right atrial; RV-FAC = right ventricular fractional area change; RV = right ventricular; RV-EDD = right ventricular end-diastolic dimension; RV-RA PG = right ventricular-right atrial pressure gradient; SIL = sildenafil; TAPSE = tricuspid annular plane systolic excursion; TR = tricuspid regurgitation.
*p<0.05 vs. the sham group; †p<0.05 vs. the MCT group.
|
RV remodeling after MCT injection as assessed noninvasively
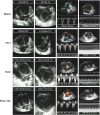 | Figure 4Representative echocardiographic images are shown for each group.LV = left ventricular; MCT = monocrotaline; MAC = macitentan; PAAT = pulmonary artery acceleration time; RV-RA PG = right ventricular-right atrial pressure gradient; SIL = sildenafil; TAPSE = tricuspid annular plane systolic excursion.
|
Invasive hemodynamic measurement
Table 1
Hemodynamic parameters 7th weeks after MCT injection





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download