1. Stern RS, Divito SJ. Stevens-Johnson syndrome and toxic epidermal necrolysis: associations, outcomes, and pathobiology-thirty years of progress but still much to be done. J Invest Dermatol. 2017; 137:1004–1008.

2. Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013; 133:1197–1204.

3. Widmer S, Grossman M. Chemotherapy patient with Stevens-Johnson syndrome presents to the emergency department: a case report. Am J Emerg Med. 2018; 36:1325.e3–1325.e4.

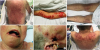
4. Karagounis T, Vallurupalli M, Nathan N, Nazarian R, Vedak P, Spring L, et al. Stevens-Johnson syndrome-like eruption from palbociclib in a patient with metastatic breast cancer. JAAD Case Rep. 2018; 4:452–454.

5. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016; 375:1738–1748.

6. Harris V, Jackson C, Cooper A. Review of toxic epidermal necrolysis. Int J Mol Sci. 2016; 17:E2135.

7. Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017; 153:514–522.

8. Faye O, Roujeau JC. Treatment of epidermal necrolysis with high-dose intravenous immunoglobulins (IV Ig): clinical experience to date. Drugs. 2005; 65:2085–2090.

9. González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al. Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. 2017; 137:2092–2100.

10. Ng QX, De Deyn ML, Venkatanarayanan N, Ho CY, Yeo WS. A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. J Inflamm Res. 2018; 11:135–142.
11. Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018; 128:985–996.

12. Gillis NK, Hicks JK, Bell GC, Daly AJ, Kanetsky PA, McLeod HL. Incidence and triggers of Stevens-Johnson syndrome and toxic epidermal necrolysis in a large cancer patient cohort. J Invest Dermatol. 2017; 137:2021–2023.

13. Gravante G, Delogu D, Marianetti M, Esposito G, Montone A. Toxic epidermal necrolysis and Steven-Johnson syndrome in oncologic patients. Eur Rev Med Pharmacol Sci. 2007; 11:269–274.
14. Rosen AC, Balagula Y, Raisch DW, Garg V, Nardone B, Larsen N, et al. Life-threatening dermatologic adverse events in oncology. Anticancer Drugs. 2014; 25:225–234.

15. Ng CY, Chen CB, Wu MY, Wu J, Yang CH, Hui RC, et al. Anticancer drugs induced severe adverse cutaneous drug reactions: an updated review on the risks associated with anticancer targeted therapy or immunotherapies. J Immunol Res. 2018; 2018:5376476.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download