CASE REPORT
A 47-year-old woman initially complained of a mass in the left anterior chest wall. On examination, a mobile, firm mass was discovered in the left breast. The patient underwent a thorough examination including a mammogram, breast ultrasonography (US), and magnetic resonance imaging (MRI), followed by a guided core needle biopsy (CNB).
The mammogram showed an isodense mass in the upper inner quadrant of the left breast (
Figure 1). Breast US revealed the presence of a 1.9 cm mass at the 10 o'clock position that appeared malignant and was most likely primary breast cancer. MRI confirmed the breast US findings. The CNB results showed that the tumor was an invasive ductal carcinoma, nuclear grade 2/3, and histological grade 2/3. No hormone receptor testing was performed on the CNB sample (
Figure 2).
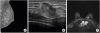 | Figure 1
Mammography, US, and MRIs of metastatic parotid ductal cancer: (A) Mammogram, (B) US image, and (C) MRI.
US = ultrasonography; MRI = magnetic resonance image.

|
 | Figure 2 Hematoxylin and eosin staining of breast and parotid tissue. (A) Breast tumor (magnification ×100), (B) Breast tumor (magnification ×200), (C) Parotid tumor (magnification ×40), and (D) Parotid tumor (magnification ×200).
|
Two years prior to her breast complaint, the patient presented with a mass on the left side of her neck that had been present for 2 months. Following radiological and pathological investigations, the mass was diagnosed as a left parotid salivary duct carcinoma (SDC) with cervical lymph node metastasis. Positron emission tomography/computed tomography (CT) revealed no distant metastases. She underwent a radical left parotidectomy with selective neck dissection, and her final pathology revealed a 3.1 cm tumor with lymphovascular and extensive facial nerve invasion; 1 of 45 excised lymph nodes was positive, and the tumor was staged as pT4a N2a M0. She received adjuvant radiotherapy and had been attending regular follow-up appointments since then. One year later, she developed lung metastasis, which was treated using stereotactic body radiation therapy.
Considering the patient's history, breast metastasis was initially considered as a differential diagnosis; however, the diagnosis was thought to be primary breast cancer after a review of radiological images and pathology samples.
She underwent breast-conserving surgery with a sentinel lymph node biopsy 2 years after her initial parotid surgery, after which the final pathology report confirmed a 2.7 cm metastatic SDC. The results of immunohistochemical staining (IHC) were negative for estrogen, progesterone, human epidermal growth factor receptor 2 receptor, and p53 but were positive for androgen receptor (AR) (
Figure 3). Her Ki-67 level was 30%. All excised lymph nodes were negative for metastases (0/8). Further IHC staining of breast and salivary tumor tissue for GATA binding protein-3 (GATA3) and gross cystic disease fluid protein-15 (GCDFP-15) was performed, both of which were positive for both markers (
Figure 4). Chest CT images showed newly developed multiple lung metastases and mediastinal lymph node enlargement, resulting in a diagnosis of SDC with multiple pulmonary, mediastinal, and breast metastases. The patient was treated with palliative chemotherapy. This case report was approved by the Institutional Review Board (IRB) of Asan Medical Center (2019-1217). The requirement for formal written informed consent was waived by the IRB.
 | Figure 3
Pathological images of samples from the parotid cancer that metastasized to the breast and the primary parotid cancer. (A) No expression of ER in the breast tumor (magnification ×200), (B) No expression of ER in the parotid tumor (magnification ×200), (C) No expression of PR in the breast tumor (magnification ×200), (D) No expression of ER in the parotid tumor (magnification ×200), (E) Positive expression of AR in the breast tumor (magnification ×200); and (F) Positive expression of AR in the parotid tumor (magnification ×200).
ER = estrogen receptor; PR = progesterone receptor; AR = androgen receptor.

|
 | Figure 4
High magnification images showing positive GATA3 and GCDFP-15 staining of both breast and salivary tumor tissues. (A) Positive GATA3 staining of breast tumor tissue (magnification ×200), (B) Positive GATA3 staining of parotid tumor tissue (magnification ×200), (C) GCDFP-15 staining of breast tumor tissue (magnification ×200), and (D) GCDFP-15 staining of parotid tumor tissue (magnification ×200).
GATA3 = GATA binding protein 3; GCDFP-15 = gross cystic disease fluid protein-15.

|
DISCUSSION
To the best of our knowledge, this is the first report of parotid SDC metastasizing to the breast. The occurrence of secondary breast malignancy is rare, and it usually originates from the contralateral breast. With the exclusion of mammary metastasis, non-mammary breast metastasis accounts for less than 2% of all breast cancers, most of which are from primary melanoma, followed by ovarian and lung cancers. Varying frequencies for each of the primary sites mentioned above have been reported by different retrospective studies. In a review of 85 cases of non-mammary breast metastasis by Delair et al. [
1], melanoma was found to account for 21% of cases, followed by ovarian and lung cancer at 16% and 13%, respectively. Other primary malignancies accounting for low numbers of cases were gastroenterological tract carcinomas (8%) and sarcomas of different types (mainly uterine) (6%), as well as a few cases of renal cell, prostatic, laryngeal and thyroid carcinomas, and testicular seminoma. There have been 2 reported cases of parotid mucoepidermoid carcinoma metastasizing to the breast [
45], but we have found no previous report of parotid SDC metastasizing to the breast.
The radiological features of breast metastasis frequently resemble those of benign breast disease, which would aid in the preoperative diagnosis of breast metastasis if interpreted alongside pathology images of the malignancy. However, some metastatic breast lesions appear similar to those of primary breast malignancy; hence, accurate preoperative diagnosis may be challenging.
On US, metastatic breast lesions appear as demarcated, round or oval, single or multiple, and, occasionally, microlobulated hypoechoic masses. However, they generally lack calcification or architecture distortion. More than half of all cases are classified as American College of Radiology's Breast Imaging Reporting and Data System (BI RADS) category 4, with a further one-fifth of cases classified as BI RADS category 3 [
46]. Often, multiple lesions are present, and these are usually bilateral. It is uncommon to have a single lesion, and when this occurs, it is difficult to distinguish it from primary breast malignancy [
78].
Mammography findings are similar to sonographic findings with a lack of microcalcifications, although there are some reported cases of ovarian, hepatocellular, and gastric cancer with breast metastasis showing microcalcifications [
79]. These lesions almost always have no speculated margins and are typically not associated with skin or nipple retractions, findings that are common in primary breast cancer. The lack of such findings can be attributed to the absence of desmoplastic reactions, which typically accompany primary breast malignancies [
56].
There are several factors that pathologically differentiate primary from secondary breast cancer, including hormone (estrogen and progesterone) staining, the presence of elastosis, calcification, and the presence of carcinoma
in situ. The presence of these differentiating factors strongly supports the diagnosis of primary breast cancer, however, their absence does not rule out its diagnosis. For example, such factors may not be present in case of triple-negative breast cancer (TNBC). Furthermore, immunophenotyping results show that ductal carcinoma of the breast (DCB) is typically cytokeratin (CK) 7 positive and CK20 negative, but this result is also shared by a few other tumors, including endometrial, thyroid, and salivary gland tumors. In addition, along with similar hematoxylin and eosin staining, the pathological appearance of parotid SDC resembles that of invasive DCB, so they are difficult to distinguish [
1011].
Other IHC methods specific to breast carcinoma, such as GATA3 and GCDFP-15 staining, are mainly used to diagnose distant metastatic tumors from breast tumors and are most useful in primary cases of TNBC. Unfortunately, these staining techniques also show some positivity for salivary gland tumors, particularly SDC, which is probably because of the similar histological form of the 2 tissues. A study of 2,040 epithelial and 460 mesenchymal or neuroectodermal neoplasms showed that 92% of primary DCB and 43% of SDC neoplasms stained positive for GATA3, whereas TNBC showed reduced GATA3 expression (40%–60%) compared with other breast cancer types [
2]. A study using anti-GCDFP-15 monoclonal antibody D6, which was applied to paraffin sections of 133 salivary neoplasms, showed that 24% of adenocarcinomas also expressed GCDFP-15 [
3].
In 1998, it was discovered that SDC was AR-positive, a characteristic usually seen in prostatic malignancy. Because of the resemblance between SDC and DCB, estrogen and progesterone receptor positivity has been widely studied in SDC and reported to be 1.3% and 6%, respectively. Surprisingly, the AR was found to be strongly positive in more than 90% of the study samples [
11]. Presently, AR positivity along with estrogen and progesterone receptor negativity is considered characteristic of SDC [
1112].
When metastasis occurs in the breast, it is commonly preceded by metastasis in another organ, and the lung is almost always involved. Metastasis to the breast is rarely a solitary event and is seldom the presenting sign of malignant disease. Therefore, the occurrence of metastasis in the breast is often a sign of late-stage disease and is treated palliatively rather that curatively. The presence of another organ metastasis was found in 77%–93% of all cases of metastasis to the breast [
147]. Another study reported that metastasis to the breast was the first sign of malignancy in only 5% of cases, and isolated breast metastasis was found in approximately 21% of cases [
4].
It should be noted that most metastases to the breasts occur ipsilateral to the primary tumors, although not all studies report the side of the body on which metastasis occurs with respect to the primary tumor. In our case, the primary tumor, the first detected lung metastasis, and the breast metastasis were all on the left side. Huang et al. [
13] reviewed 6 cases of lung adenocarcinoma that developed breast metastasis over a 10-year period in a tertiary hospital. All cases had breast metastasis on the same side as the primary lung adenocarcinoma. In addition, all of these cases shared ipsilateral pleural effusion or thickness and axillary lymph node enlargement. This led to a hypothesis regarding the mode of metastasis, according to which lung cancer cells spread to axillary lymph nodes retrogradely via intercostal and/or supraclavicular lymph nodes and then to the breast via the intramammary lymphatics [
13]. Nevertheless, axillary lymph node involvement is less common in secondary compared with primary breast malignancy, which would contradict the above-mentioned theory [
1415]. Taking into consideration the rare occurrence of breast metastasis, this can be mainly attributed to the histological nature of breast tissue, which has abundant fat and connective tissue and minimal blood supply [
14].
The prognosis of breast metastasis is poor, and the average survival after diagnosis is approximately 15 months. As breast metastasis is usually preceded by metastasis elsewhere, multiple and disseminated metastases are common, which explains the poor prognosis of the disease. Hence, it is important to differentiate primary from secondary breast malignancy early to spare the patient the burden of unnecessary procedures [
1].
In conclusion, metastasis to the breast is a rare event. To our knowledge, there are no previously reported cases of parotid SDC metastasis to the breast. In our case, the breast lesion resembled primary breast cancer both radiologically and pathologically. Although it was difficult to rule out TNBC by IHC itself because of the similarity of its features with SDC, after considering the clinical presentation and postoperative pathologic findings, which were the same as those for primary SDC, a diagnosis of SDC metastasis to the breast was made.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download