This article has been
cited by other articles in ScienceCentral.
Abstract
Nipple-areolar skin-sparing mastectomy followed by autologous reconstruction in patients with large, ptotic breasts often offers a limited field, resulting in strenuous traction. Skin-sparing mastectomy (SSM) with immediate nipple grafting from the specimen was attempted for such patients. Patients who underwent SSM with immediate autologous breast reconstruction and nipple grafting between September 2016 and February 2019 were evaluated, including 33 nipple grafts in 30 patients. The average weight of the mastectomy specimen was 552.5 g and the average operation time for unilateral mastectomy was 109 minutes. No complete nipple loss or major skin flap necrosis was reported. Adjuvant therapy started after an average of 24 days. SSM with immediate nipple grafting on the autologously reconstructed breast could be an alternative for large, ptotic breasts. It is also useful for patients requiring contralateral balancing procedures or those with bilateral breast cancer in which only one nipple can be spared oncologically.
Keywords: Breast neoplasms, Free tissue flaps, Mammaplasty, Subcutaneous mastectomy
Skin-sparing mastectomy (SSM) or nipple-areolar skin-sparing mastectomy (NASSM) followed by immediate reconstruction provide excellent aesthetic results and high patient satisfaction. However, spared skin does not always guarantee complete survival. Ito et al. [
1] reported 7% and 13% incidences of skin flap or nipple-areolar complex (NAC) necrosis after skin-sparing or NASSM, respectively. The risk factors for skin or NAC necrosis include obesity and large breast size [
12]. NASSM for large, ptotic breasts requires dissection of a large area under a limited visual field. In free flap reconstruction, restricted access to the recipient vessels (usually the internal mammary artery and vein) necessitates strenuous traction or additional incisions, which further jeopardize mastectomy flap circulation. Periareolar or circumareolar incisions are associated with an increased incidence of nipple necrosis [
34]. Yang et al. [
5] raised concerns that mastectomy flap traction during recipient vessel preparation and microanastomoses might adversely affect skin flap and NAC viability.
Endara et al. [
3] reported an association between autologous tissue reconstruction and increased incidence of nipple necrosis (17.3%) compared to that for prosthesis-based reconstruction (4.1%–4.5%). This may be partly because autologous reconstruction is commonly used for women with larger breasts and a higher body mass index [
6]; however, the influence of the above-mentioned factors should also be considered. Necrosis of the spared nipple in NASSM results in aesthetically poor results such as loss of projection and depigmentation. It may also necessitate the removal of the necrotized nipple [
78]. Moreover, major mastectomy flap necrosis occasionally requires additional surgery or delay of adjuvant therapies.
Composite grafting of the nipple is a classic method for nipple reconstruction when it cannot be spared during total or partial mastectomy. We have performed SSM and immediate nipple grafting from the ipsilateral NAC on the autologously reconstructed breast in patients with relatively large and ptotic breasts even if the NAC could be spared oncologically. The protocol in the present study included women with intermediate to large (> 400 g) breasts and significant ptosis who agreed and provided informed consent to undergo the procedure. The study was approved by the Institutional Review Board of the Asan Medical Center (2018-1329). Since this surgical technique is within the standard of care, patients' consent for the study protocol was exempt, except for the routine informed consent for postmastectomy breast reconstruction and nipple graft procedures.
Mastectomy was performed using typical SSM procedures with either radial extension or symmetrical designs with the contralateral side when balancing procedures such as reduction/mastopexy were planned. The NAC was separated from the mastectomy specimen and the nipple was severed using a No.10 blade (
Figure 1A). Frozen biopsy samples were obtained from the base of the nipple. After autologous reconstruction, the center of the skin paddle was de-epithelized to a diameter of approximately 1–1.5 cm (
Figure 1B). The nipple graft was sutured using 5-0 Vicryl Rapide
® (polyglactin 910) absorbable sutures (Ethicon Inc., New Jersey, USA) in a simple interrupted manner with slightly greater tension than that for typical skin sutures (
Figure 1C). Ointment and commercially available foam dressings were used to keep the grafted nipple moist at all times. Most of the nipples proceeded through a similar typical clinical course, with initial paleness for the first few (3–5) days, followed by a congestive period of 1–2 weeks before the development of neoangiogenesis from the de-epithelialized bed (
Figure 1D and E). Nipple swelling was noted during the congestive period because venous and lymphatic function fully recovered after arterial inosculation. Final stabilization of the grafted nipple typically occurred at between 10 and 20 days.
This technique was considered in patients with large (estimated mastectomy specimen weight ≥ 400 g) or ptotic (ptosis grade ≥ 2, or nipple > 1 cm below the level of the inframammary crease) breasts who provided informed consent. Thirty-three immediate nipple grafts were performed in 30 patients between September 2016 and February 2019 (
Figure 2A and B). Among these, 13 patients underwent mastopexy or reduction of the contralateral breast due to large and/or ptotic breasts. The average weight of the mastectomy specimen was 552.5 ± 148.5 (383–1,000) g. The average operation time was 109 ± 30 (55–160) minutes for unilateral mastectomy. Adjuvant chemotherapy or radiation therapy was required in 17 patients and started after an average of 24 (11–36) days. Patient demographics, operation data, and treatments are summarized in
Table 1.
No complete nipple loss or major skin flap necrosis was reported and all nipples survived. Partial necrosis of the nipple tip was observed in several cases. However, all partial loss was managed conservatively and recovered after shedding of the necrotic eschar. Two patients underwent wound repair at an outpatient clinic due to minor skin flap necrosis outside the NAC.
Ito et al. [
1] reported that compared to SSM, NASSM was a significant risk factor for skin flap and/or NAC necrosis, in addition to the weight of the breast resection. Frey et al. [
2] demonstrated that NASSM in large breast group patients was significantly more likely to be associated with major mastectomy flap necrosis and complete or partial NAC necrosis when compared to those in groups of patients with intermediate or small breasts. Frey et al. [
6] reported incidences of 7.7% and 5.8% for minor mastectomy flap and partial nipple necrosis, respectively, among more than 1,000 NASSMs. They reported NASSM to be a procedure with a significant learning curve and emphasized the importance of careful patient selection.
SSM incision with radial extension offers an adequate visual field and access to the whole breast, including the axilla. This procedure is less technically demanding and time-consuming for both breast and plastic surgeons in patients with large, ptotic breasts. van Verschuer et al. [
9] showed better satisfaction with breast appearance and overall outcome for SSM compared to those for NASSM, whereas NAC-specific satisfaction was comparable between the two groups. Loss of projection, depigmentation, malposition, or sensory loss of sparing-attempted nipple can result in dissatisfaction.
Mastroianni et al. [
7] reported that 6.6% of nipples were removed afterward due to oncologic reasons (49%) or necrosis (35%). Dent et al. [
8] reported a sensitivity of 64% for frozen sections, which increased to 75% when suspicious and atypical findings were included. Rarely, areolar disease may present in cases of isolated nipple excision [
8]. Our method generally provided a sufficient specimen for nipple margin and areola without concern for disrupting nipple circulation, although areolar tattooing is required afterward.
One may wonder whether the conversion from NASSM to SSM with nipple grafting was decided in the operating theater because of questionable survival of the mastectomy flap or if it was predetermined according to the breast size and ptosis. We always fully discussed the options with patients who we believed to be good candidates (about > 400 g of breast with significant ptosis) to facilitate the surgical procedure and possibly reduce operation time and mastectomy flap-related complications.
SSM with immediate nipple grafting is especially useful for patients who are candidates for contralateral reduction or mastopexy because adjusting the nipple position after NASSM is technically demanding and hazardous in terms of circulation. SSM with skin reduction and immediate nipple graft offers a safe opportunity to move the nipple to the desired position (
Figure 2C and D).
The success of the grafted nipple depends on the development of neoangiogenesis from the de-epithelized bed. The autologous tissue flap provides adequate initial plasma supply and allows for neoangiogenesis. However, nipple grafting in prosthesis-based reconstruction is not recommended since the perfusion of the mastectomy flap is usually not sufficient to ensure graft survival.
In conclusion, SSM with immediate nipple graft in autologous tissue reconstruction provided excellent visual field and access for both breast and plastic surgeons in women with large, ptotic breasts. This procedure also provided a safe opportunity to control the nipple position. We recommend this technique as an alternative to conventional NASSM mainly for patients with significantly large, ptotic breasts, patients with risk factors for NAC necrosis, or patients requiring contralateral balancing procedures. The long-term oncologic consequences of this method should be explored and compared to those of conventional NASSM.
Figures and Tables
Figure 1
The procedure of immediate nipple grafting and the progression of graft survival (A) After completion of skin-sparing mastectomy, the nipple-areolar complex was separated from the specimen, and the nipple was severed. Frozen biopsy samples were obtained from the base. (B) The center of the flap skin paddle was de-epithelized to a diameter of 1 to 1.5 cm. (C) Composite nipple graft was sutured using 5-0 absorbable sutures in a simple interrupted manner with slightly greater tension than typical skin sutures. (D) Nipple was typically pale on the second postoperative day. (E) Congestion developed 7 days after surgery.
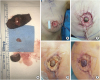
Figure 2
Follow up photographs of our patients. (A) A 50-year-old female patient had diffusely spreading ductal carcinoma in situ on her left breast. Skin-sparing mastectomy with immediate nipple grafting and free DIEP flap reconstruction was done. Postoperative 11 months photograph, after completion of tattooing. (B) A 62-year-old female patient with ductal carcinoma in situ on her left breast underwent skin-sparing mastectomy with immediate nipple grafting and free TRAM flap reconstruction. Postoperative six months photograph, before tattooing. (C and D) A 61-year-old female patient with invasive ductal carcinoma with microcalcifications on her left breast underwent skin-sparing mastectomy (560 g) with immediate nipple grafting, and immediate reconstruction was done by free TRAM flap with contralateral mastopexy. Pre- and 6 months post-operative clinical photographs.
DIEP = deep inferior epigastric perforator; TRAM = transverse rectus abdominis musculocutaneous.
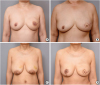
Table 1
Patients demographics, operation data, and treatments

|
Characteristics |
Values |
|
Mean age (yr) |
48.4 ± 6.6 |
|
≤ 50 |
18 |
|
> 50 |
12 |
|
Mean BMI (kg/m2) |
26.6 ± 3.2 |
|
≤ 23.5 |
7 |
|
> 23.5 |
23 |
|
Mean mastectomy specimen weight (g) |
552.5 ± 148.5 |
|
≤ 400 |
2 |
|
> 400 |
31 |
|
Mean operation time (min)*
|
109 ± 30 |
|
≤ 120 |
20 |
|
> 120 |
7 |
|
Axillary dissection |
|
|
SLNB only |
25 |
|
ALND |
8 |
|
cT stage |
|
|
in situ, 1 |
18 |
|
2 |
11 |
|
3, 4 |
4 |
|
cN stage |
|
|
0 |
22 |
|
≥ 1 |
11 |
|
Neoadjuvant chemotherapy |
|
|
Yes |
9 |
|
No |
21 |
|
Adjuvant chemotherapy |
|
|
Yes |
8 |
|
No |
22 |
|
Adjuvant endocrine therapy |
|
|
Yes |
19 |
|
No |
11 |
|
Adjuvant trastuzumab therapy |
|
|
Yes |
8 |
|
No |
22 |
|
Adjuvant radiation therapy |
|
|
Yes |
10 |
|
No |
20 |







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download