INTRODUCTION
Approximately 5%–10% of breast cancer patients are carriers of at least one breast cancer susceptibility gene, including
BRCA1,
BRCA2,
TP53,
PTEN,
LKB1, and
MSH2/MLH1 [
1], and most hereditary breast cancers are derived from
BRCA1 and
BRCA2 mutations [
2]. Women with a germline mutation in either the
BRCA1 or
BRCA2 gene have a high-risk of developing breast cancer over their lifetime [
345]. One meta-analysis showed that women with
BRCA1 and
BRCA2 mutations had 57% and 49% risks of developing breast cancer by the age of 70 years, respectively [
4]. Compared to patients with sporadic breast cancer, women with
BRCA1- and
BRCA2-associated breast cancer are reported to have 4.5- and 3.4-fold increased risks of contralateral breast cancer (CBC), respectively [
6]. In addition, several studies have shown a higher risk of ipsilateral breast tumor recurrence (IBTR) in patients with
BRCA-associated breast cancer treated with breast-conserving surgery (BCS) than in sporadic controls who received BCS [
78910].
The Korean Hereditary Breast Cancer study, a large prospective nationwide study to estimate the prevalence of
BRCA1/2 mutations and ovarian cancer among a high-risk group of patients with hereditary breast cancer and their families, has reported average cumulative risks of breast cancer of 72.1% and 66.3% among
BRCA1 and
BRCA2 mutation carriers, respectively, by the age of 70 years [
511]; furthermore, the risks of CBC at 5 years after primary breast cancer were 16.2% and 17.3%, respectively.
The purpose of this study was to assess the risks of CBC and IBTR and to determine the predictive factors for CBC and IBTR among invasive breast cancer patients at high-risk of hereditary breast and ovarian cancer (HBOC) according to the germline mutations of the BRCA1/2 genes.
METHODS
We analyzed prospectively collected cohort data of 772 patients at high-risk of HBOC who underwent genetic testing for
BRCA1/
2 mutations between 2003 and 2013 at Seoul National University Bundang Hospital. The indication for
BRCA testing was as follows: 1) having at least one third-degree relative with breast or ovarian cancer, 2) young breast cancer patients (age at diagnosis ≤ 40 years), 3) breast cancer patients with multiple primary cancers, 4) male breast cancer patients, and 5) breast cancer patients from a family with known
BRCA mutations. Among those who received genetic testing for BRCA1/2 mutations, study subjects included patients who underwent curative surgery for invasive breast cancer. Patients with synchronous bilateral breast cancer were excluded because the purpose of this study included an evaluation of the risk of CBC. We also excluded patients who underwent total mastectomy when analyzing the risk of IBTR (
Figure 1).
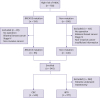 | Figure 1
Diagram for case enrollment.
HBOC = hereditary breast and ovarian cancer; CBC = contralateral breast cancer; IBTR = ipsilateral breast tumor recurrence.

|
Data on age at diagnosis, tumor size, nodal status, histologic grade of tumor, estrogen receptor (ER) status, human epidermal growth factor receptor-2 (HER2) expression, operation methods, chemotherapy, radiotherapy, bilateral oophorectomy, familial tree for breast cancer, and information on CBC and IBTR were collected from medical records. CBC was defined as a newly diagnosed tumor in the contralateral breast after treatment of the first breast cancer. IBTR was defined as all events that occurred in the ipsilateral remnant breast tissue after BCS. We did not distinguish between true recurrence and new primary cancer, but any ipsilateral event within 6 months after surgery was considered a local failure and excluded from IBTR.
Written informed consent was obtained from all patients, and this study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-1701/377-102).
Statistical analysis
The date of the last follow-up was defined as the date of last contact or death. The Kaplan-Meier product-limit method was used to calculate the cumulative risks of developing CBC or IBTR after the first breast cancer, while the log-rank test was used to compare the cumulative risks between groups. We also compared the risk of developing CBC and IBTR according to tumor characteristics (ER, HER2, and nodal status), the number of first-degree relatives with breast cancer, and the type of treatment received including surgery, chemotherapy, radiotherapy, hormone therapy, and oophorectomy. In these analyses, oophorectomy was treated as a time-dependent covariate. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). IBM SPSS Statistics version 22 (IBM Corp, Armonk, NY, USA) was used for cumulative risks, and R version 3.3.2 (
http://www.R-project.org/) was used for the HRs. A
p-value < 0.05 was considered statistically significant.
DISCUSSION
Women with a history of breast cancer are known to have an increased risk for CBC. According to the Surveillance, Epidemiology, and End Results database, breast cancer survivors have an approximately 1.5- to 2-fold increased risk for subsequent CBC compared to the risk of developing breast cancer in the general population [
12]. Furthermore, compared to patients with sporadic breast cancer, women with
BRCA1- and
BRCA2-associated breast cancer have 4.5- and 3.4-fold increased risks of CBC, respectively [
6]. The risk of CBC in patients with
BRCA-associated breast cancer is 1.5%–3.1% annually, with 10-year estimates of 25%–38% for mutation carriers from high-risk families compared with a 3%–7% rate for women without mutations [
678]. Metcalfe et al. [
13] reported that the 15-year actuarial risk of CBC was 36.1% for
BRCA1 mutation carriers and 28.5% for
BRCA2 mutation carriers. In their study, the 10-year risk of CBC was 23.8% for women with a
BRCA1 mutation and 18.7% for those with a
BRCA2 mutation [
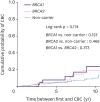
13], which is consistent with our results.
Meanwhile, the 10-year risk of CBC in women with sporadic breast cancer was reported to be about 5%, which was significantly lower than that in
BRCA mutation carriers [
1415]. In the current study, the 10-year risk of CBC in non-carriers at high-risk of HBOC was 9.8%, which is approximately 2 times higher than in those with sporadic breast cancer. A report from the Women's Environment, Cancer and Radiation Epidemiology study supports our results and demonstrates 10-year cumulative risks of CBC for non-carriers with no family history, second-degree family history only, a first-degree family history, and a history of bilateral breast cancer in a first-degree relative of 4.6%, 5.9%, 8.6%, and 15.6%, respectively [
15].
Several factors are reported to influence the risk of CBC in
BRCA mutation carriers with breast cancer. Younger age at the time of first breast cancer diagnosis is reported to be associated with a higher risk of CBC in patients with a
BRCA mutation [
613]. Malone et al. [
6] reported that the relative risk (RR) of CBC for
BRCA mutation carriers increased as the age of first diagnosis increased. Metcalfe et al. [
13] reported that women aged younger than 50 years are at significantly higher risk of developing CBC in 15 years than those aged older than 50 years. In the current study, however, we could not analyze the risk factors of CBC according to the
BRCA mutation status because the number of patients who developed CBC in each group was too small; therefore, we analyzed all patients who were at high-risk of HOBC irrespective of the
BRCA mutation. Thus, in the current study, age < 50 years was not significantly associated with an increased risk of developing CBC, although the risk of CBC tended to be higher in younger patients than in older patients.
Previous studies have found a 1.3- to 1.8-fold higher risk of CBC in
BRCA1 mutation carriers than in
BRCA2 mutation carriers [
61617]. We showed that patients with
BRCA1/
2 mutations have a significantly higher risk of developing CBC than do high-risk non-carriers, though we failed to show any significant difference in the risk of developing CBC between
BRCA1 and
BRCA2 carriers. This is probably due to the limited sample size and inadequate length of follow-up. In Metcalfe's study [
13], the ER status of primary cancer was not associated with the risk of CBC. However, in general, patients with ER-positive tumors are considered to be at decreased risk for CBC [
18]. In our study, negative ER status of the primary tumor was found to be a significant predictive factor for increased risk of CBC, probably because we analyzed the risk factors of CBC by combining both
BRCA mutation carriers and high-risk non-mutation carriers.
BCS combined with radiotherapy is a standard treatment for early-stage breast cancer and yields a survival rate equivalent to that in mastectomy in women with sporadic breast cancer [
1920]. Among breast cancer patients treated with BCS,
BRCA mutation carriers have a reportedly higher risk of ipsilateral in-breast events, including IBTR and the development of a second primary tumor, compared to sporadic controls [
78910]. In a multi-institutional study by Pierce et al. [
8], the 10-year rate of IBTR was twice as high among
BRCA1/
2 mutation carriers treated with BCS than among sporadic controls who received BCS. Another multi-institutional study demonstrated that compared with
BRCA mutation carriers treated with mastectomy, carriers who underwent BCS had an elevated risk of local failure in the ipsilateral breast, most occurrences of which appeared to be second primary cancers rather than failure to control the primary tumor [
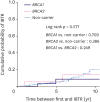
21]. Metcalfe et al. [
22] also reported a 10-year risk of IBTR of 11% for women with a
BRCA1 mutation and 17% for women with a
BRCA2 mutation, which closely corresponds to our results. However, we found no significant difference in the development of IBTR between
BRCA1/
2 mutation carriers and high-risk non-carriers, which was likely because we compared
BRCA mutation carriers with high-risk non-carriers and not with sporadic breast cancer patients. In the study by Kirova et al. [
23], there was also no difference in the risk of IBTR between
BRCA mutation carriers and non-carriers with a family history of breast and/or ovarian cancer.
The chief treatment goal in patients with breast cancer is to minimize the risk of death from primary breast cancer. For women with
BRCA-associated breast cancer, however, minimizing the incidence of and mortality due to subsequent cancers, such as metachronous CBC and ovarian cancer, is equally as important as treating the primary breast cancer. While intensified screening may help identify subsequent cancers at an early and favorable stage, it cannot prevent the development of such cancers. Therefore, preventive strategies, such as contralateral prophylactic mastectomy (CPM), prophylactic oophorectomy (PO), or chemoprevention with tamoxifen may be considered in patients with
BRCA mutations to reduce their risk for developing subsequent cancer. CPM has been reported to reduce the risk of CBC by at least 90% [
24], and a meta-analysis has shown that PO is associated with a decreased risk of CBC in patients with
BRCA1/
2-associated breast cancer (RR, 0.52; 95% CI, 0.37–0.74) [
25]. Tamoxifen also decreased the incidence of CBC [
1626], and its use was associated with a 50% reduction in the risk of CBC in
BRCA mutation carriers independent of the protective effect of PO [
26].
PO may also help reduce the risk of IBTR in patients with
BRCA-associated breast cancer treated with BCS. Pierce et al. [
8] have shown that the rates of IBTR were twice as high among
BRCA1/2 mutation carriers who did not undergo PO than among sporadic controls, though the rates were similar between mutation carriers who underwent PO and sporadic controls. Metcalfe et al. [
22] also reported that PO was associated with a significant reduction in the risk of IBTR in patients with
BRCA mutations, particularly for
BRCA1 mutation carriers. In the study by Pierce et al. [
8], the use of tamoxifen was associated with a 63% reduction in IBTR among patients who did not undergo PO. Moreover, although data on the survival of
BRCA-associated breast cancer patients who opt for subsequent CPM are inconsistent [
27], PO appears to be associated with improved breast and ovarian cancer-specific mortality as well as improved all-cause mortality among
BRCA1/
2 mutation carriers [
28].
Although we assessed a prospectively collected cohort data of patients with
BRCA mutations at high-risk of HBOC, a study limitation is its single-center, retrospective design. The small number of patients in each subgroup resulted in weak statistical power and a wide CI. Furthermore, all of the patients assessed were Korean and represented an ethnically homogenous Far East Asian population; however, the characteristics of Korean patients with
BRCA mutation-associated breast cancer are not different from those of Western patients [
29]. Another potential limitation is the insufficient follow-up period; follow-up should span more than 10 years since the risk of developing CBC or IBTR in
BRCA mutation carriers continues to increase over time, as do the differences in the risk levels among sporadic patients [
78]. In the current study, we were unable to observe a reduction in CBC and IBTR associated with oophorectomy, probably because the preventive effect of oophorectomy among
BRCA1/
2 mutation carriers is only evident 15 years after the procedure [
30]. In this study, the number of younger patients was higher among
BRCA1 mutation carriers than among
BRCA2 mutation carriers and non-carriers. However, the effect of age was not considered when calculating the cumulative risk, which could be a weakness of this study.
In conclusion, the results of this study show that both BRCA1/2 mutation carriers and non-carriers at high-risk of HBOC are at high-risk of developing CBC and IBTR. Our results emphasize the importance of careful history taking, including a detailed family history, to determine whether the patient is at high-risk of HBOC. Counseling for preventive measures, regardless of BRCA1/2 mutation status, should be considered in such patients. Further preventive strategies to reduce the risk of CBC and IBTR in BRCA mutation carriers and non-carriers with a high-risk of HBOC should be investigated.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download