Abstract
Purpose
We report a case of optic neuropathy in a patient who was treated with pre-extensively drug-resistant tuberculosis(pre-XDR TB) treatment with linezolid.
Case summary
A 61-year-old male patient with no other underlying disease was diagnosed with pre-XDR Tb 6 months before and took the TB drug at another hospital. Ethambolol was not prescribed because it was resistant from the beginning of TB treatment. Threrefore, linezolid was included for treatment of pre-XDR TB. The patient's best corrected visual acuity was 20/400 in both eyes at the time of outpatient visit. In Ishihara color vision test, both eyes showed complete color blindness. There was no detectable relative afferent pupillary defect, and fundus examination showed hyperemic optic discs and visual field examination showed both central visual field defects. Linezolid induced optic neuropathy was suspected, the drug was discontinued. After one month, the patient's best corrected visual acuity recovered to 20/20 in both eyes, and visual field and color vision returned to normal at 3 months.
Conclusions
Linezolid is a broad spectrum antibiotic and is a useful drug for the treatment of broad-spectrum tuberculosis. However, since long-term use may cause optic neuropathy, the possibility of optic neuropathy should always be considered. If optic neuropathy is suspected, prompt drug withdrawal is required and reversible clinical changes can be expected.
Figures and Tables
Figure 1
Initial FP, VF, and OCT findings. (A) FP shows slightly hyperemic disc on both eyes. (B) VF shows both central scotomaand left eye scomta is bigger than right eye. (C) OCT shows edematous RNFL on both eyes. FP = fundus photo; VF = visual field;OCT = optical coherence tomography; RNFL = retinal nerve fiber layer; S = superior; N = nasal; I = inferior; T = temporal.
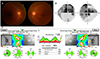
Figure 2
Initial macular OCT findings and initial VEP test. Arrows (A, B) show normal ellipsoid zone on both eyes. (C) PatternVEP test shows slightly decreased P100 amplitude on both eyes. OCT = optical coherence tomography; VEP = visual evokedpotential.
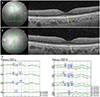
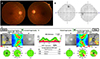
Figure 3
Follow up of FP, VF, and OCT. Three months later after treatment, (A) FP and (B) VF show normalized fundus and visualfield on both eyes. (C) OCT shows RNFL swelling is reduced to normal range on both eyes. FP = fundus photo; VF = visual field;OCT = optical coherence tomography; RNFL = retinal nerve fiber layer; S = superior; N = nasal; I = inferior; T = temporal.
References
1. Thompson MJ, Albert DM. Ocular tuberculosis. Arch Ophthalmol. 2005; 123:844–849.
2. Yoon YS, Lee SH, Min JK, Lee CK. A case of spontaneous recovery of an iris cyst in a patient with peritoneal tuberculosis. J Korean Ophthalmol Soc. 2018; 59:491–495.
3. Mok JH. Updates of tuberculosis diagnosis and treatment. J Korean Soc Health Syst Pharm. 2016; 33:101–110.
4. Pilania RK, Arora A, Agarwal A, et al. Linezolid-induced mitochondrial toxicity presenting as retinal nerve fiber layer microcysts and optic and peripheral neuropathy in a patient with chronic granulomatous disease. Retin Cases Brief Rep. 2018; 07. DOI: 10.1097/ICB.0000000000000777. PMID30048406.
5. Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012; 367:1508–1518.
6. Yoon SW, Choi JC. Treatment of pulmonary tuberculosis. J Korean Med Assoc. 2019; 62:25–36.
7. Kang YA. Diagnosis and treatment of multidrug-resistant tuberculosis. J Korean Med Assoc. 2014; 57:27–33.
8. Garrabou G, Soriano À, Pinós T, et al. Influence of mitochondrial genetics on the mitochondrial toxicity of linezolid in blood cells and skin nerve fibers. Antimicrob Agents Chemother. 2017; 61:e00542–e00517.
9. De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006; 42:1111–1117.
10. Park SJ, Jung JU, Kang YK, et al. Toxic optic neuropathy caused by chlorfenapyr poisoning. J Korean Ophthalmol Soc. 2018; 59:1097–1102.
11. Wang L, Dong J, Cull G, et al. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Invest Ophthalmol Vis Sci. 2003; 44:2–9.
12. Perry CM, Jarvis B. Linezolid: a review of its use in the management of serious gram-positive infections. Drugs. 2001; 61:525–551.
13. Ishii N, Kinouchi R, Inoue M, Yoshida A. Linezolid-induced optic neuropathy with a rare pathological change in the inner retina. Int Ophthalmol. 2016; 36:761–766.
14. Rho JP, Sia IG, Crum BA, et al. Linezolid-associated peripheral neuropathy. Mayo Clin Proc. 2004; 79:927–930.
15. Park DH, Park TK, Ohn YH, et al. Linezolid induced retinopathy. Doc Ophthalmol. 2015; 131:237–244.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download