Abstract
Background
Genetic counseling (GC) provides many benefits, including the identification of patients appropriate for testing, patient education, and medical management. We evaluated the current status of and challenges faced by GC practitioners in Korean hospitals.
Methods
An electronic survey was designed and conducted in 52 certified laboratory physicians belonging to the Korean Society of Laboratory Medicine, from August to September 2018. The questionnaires addressed three main categories of information: (1) current status of GC in hospitals; (2) essential qualifications of GC practitioners; and (3) challenges and perspectives for GC. Fisher's exact test was applied to analyze categorical data.
Results
Among a total of 52 participants who initially responded, 12 (23.1%) were performing GC either by direct or indirect care. GC clinics were opened regularly for one (33.3%) or more than three sessions (25.0%) per week; most respondents spent more time for pre-visit activities than in-person visits, both for a initial visit patient and for a follow-up visit patient. All laboratory physicians provided genetic information to their patients. Most recommended family genetic testing when indicated (91.7%), discussed disease management (75.0%), and/or ordered additional genetic testing (58.3%), and some referred patients to other specialists (8.3%).
Conclusions
Both patients and laboratory physicians concede the advantage of GC performed by clinical geneticists; however, the practice of GC involves several challenges and raises some concerns. The cost and support required to implement GC need to be addressed in order to provide qualified GC in Korea.
Health care is increasingly moving towards personalized or precision medicine, which involves the use of genetic or genomic information to guide decision-making with regard to disease prevention, diagnosis, and treatment. To date, personalized medicine has mainly been integrated into oncology and the management of disease through pharmacogenomics [1]. In addition, the diagnosis of rare genetic disorders has been achieved more readily through the advancement of molecular techniques, with several successful cases of treatment using personalized or targeted therapies [2]. In line with the promise of personalized medicine, the demand for genetic testing has increased rapidly in Korea since 2017, when the costs of targeted next-generation sequencing (NGS) panels for inherited disorders and malignancies were first covered nationally [3].
As genetic testings are expensive, time-consuming, and may be performed only once in a patient's lifetime, their use should be carefully considered and optimized to provide the greatest benefit for patients. Genetic testings have been found to be cost-effective when combined with genetic counseling (GC), the economic and societal impact of which have been well-studied [45]. Complete GC services provided by a clinical geneticist are more cost-effective than brief counseling provided by a general physician; appropriate patient communication depends on the physician's understanding of genetic testing procedures, results, and the implications thereof [67].
Patients are largely in favor of GC, especially for rare genetic disorders, although a substantial proportion of patients or families in Korea have not undergone GC and have no access to relevant information [8]. Primary-care physicians and non-genetic specialists are often uncomfortable communicating the results of even a single gene testing to their patients or have some negative bias towards the value of genetic testing for their patients [7]. Additional barriers to the use of genetic testing results in clinical care include limited understanding of genomic information, confusion regarding terminology, and the volume of information arising from genetic testing [9].
With an increase in demand for GC in clinical settings, we evaluated the current status of and challenges faced by GC practice in Korean hospitals. To our knowledge, this study is the first to examine current GC practice in Korea.
As the majority of genetic services in Korea are provided and managed by departments of laboratory medicine, a national volunteering electronic survey was conducted in 108 certified laboratory physicians belonging to the Korean Society of Genetic Diagnostics including working members of non-teaching or teaching hospitals [10]. During August 2018, the survey was planned and the questionnaires were critically reviewed by experts within the Korean Society for Genetic Diagnostics genetic counseling committee. The data were collected using a prospective cross-sectional survey and merged in September 2018. The survey addressed three main categories of information: (1) current status of GC in hospitals; (2) essential qualifications of GC practitioners; and (3) challenges and perspectives of GC. Categories 1 and 2 were addressed through closed-ended questionnaires, and information for Category 3 was gathered through an open-ended question in the survey. This study was exempted for approval by the Institutional Review Board of National Health Insurance Service, Ilsan Hospital, since it was anonymous and participation was voluntary.
Reponses were calculated as a percent of total respondents of each questionnaire. Statistical analyses were performed using R software, version 3.3.3, 64 bit (The R Foundation of Statistical Computing, Vienna, Austria). Fisher's exact test was used to determine whether the characteristics of hospitals performing GC differed or not. A two-tailed P<0.05 was considered statistically significant.
The characteristics of the 52 respondents (survey response rate of 48.1%) are summarized in Table 1. Twelve respondents (23.1%) were performing GC either by direct or indirect care, such as participating as members of the multidisciplinary team or clinical support, and 40 respondents (76.9%) were not yet performing GC. The GC service differed among different-sized hospitals but did not differ significantly among service type (P>0.05). GC service did not significantly differ among geographical regions and did not vary by number of working laboratory physicians; however, the service was provided in all teaching hospitals.
Of the 12 respondents providing GC services, 10 had outpatient clinics for GC, and eight were providing GC by consultation. Most respondents reported 10 or fewer GC cases per month (66.7% of 12 respondents), while 16.7% of them reported 51–100 patients per month and clinics were open regularly. Most respondents spent 30–59 minutes on pre-visit activities prior to a patient's initial visit, whereas the time spent with patients during follow-up visits varied from <30 to 59 minutes. The time spent for in-person visit activities on new patients (such as collection of medical and family history, running risk models for the patient or family members, educating about genetics and conditions, explaining genetic testing results, and providing psychosocial support) was 15–30 minutes for nine respondents (75.0% of 12 respondents), and 15–30 minutes were spent on patient follow-up (58.3%; Table 2).
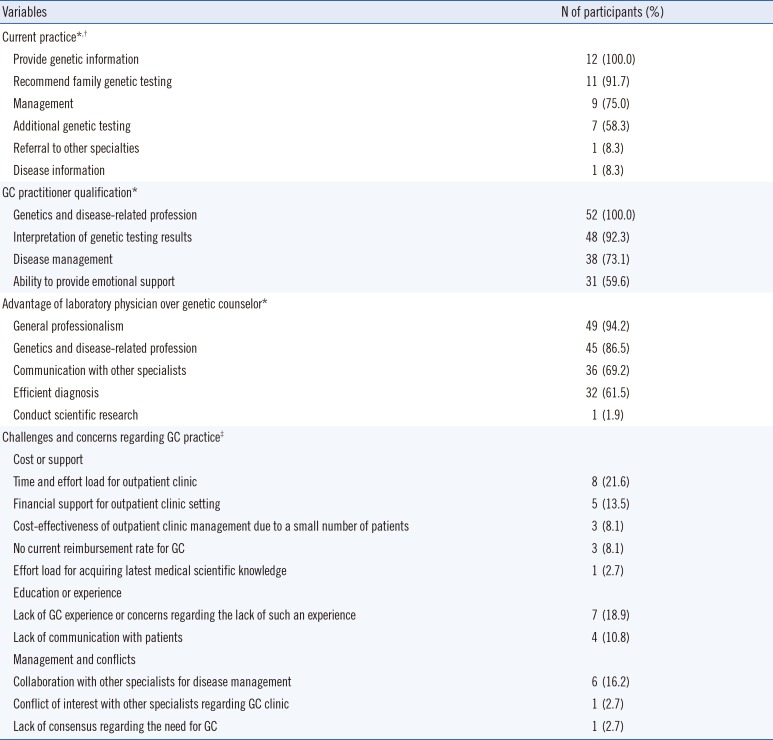
All respondents reported providing genetic information to their patients, recommending family genetic testing when indicated (91.7%), discussing disease management (75.0%), ordering additional genetic testing (58.3%), and referring patients to other specialists (8.3%). Further, they indicated that professional training in genetics and disease is essential, and the capacity to provide emotional support (59.6%) and information regarding the interpretation of genetic testing results (92.3%) and disease management (73.1%) is important in order to offer GC to patients. Majority of respondents considered general professionalism, knowledge of genetics and diseases, efficient provision of diagnoses, and the ability to communicate with other specialists and conduct scientific research can be the advantages of laboratory physicians related to GC (Table 3).
Although 94.2% of laboratory physicians conceded the advantages of GC performed by a clinical geneticist, we identified several challenges and concerns regarding GC practice that can be categorized into the cost and support required to implement GC (Table 3).
GC provides many benefits to patients, including the identification of patients appropriate for testing, patient education, and appropriate medical management [11]. From the data collected in 2016, it has been estimated that there is only one genetic counselor per 89,324–16,390,000 residents (median 1:2,000,000) and one clinical geneticist per 262,867–10,930,000 residents (median 1:2,480,000) in the Asia-Pacific region, [12]; it is estimated that there are 4,242 certified genetic counselors in the United States [13]. In Korea, approximately 80 and 30 genetic counselors were trained through a GC program offered by the Korean Society of Genetic Diagnostics and the Korean Board of Medical Genetics, respectively; however, only a limited number of clinicians can offer GC and only for certain disorders [8]. As GC services are not currently well recognized to be a valuable part of clinical genetics services in Korea, residency programs for training clinical geneticists are yet to be organized, and significant workforce shortages are expected in the near future.
Despite the general lack of genetic counselors, patients in other countries report being satisfied with the emotional ramifications of going through GC and the help they receive in making important decisions when the length of the counseling session is approximately 90 minutes [14], whereas in Korea the mean duration of in-person visits is generally 6.2 minutes in outpatient clinics [15]. In addition to the time spent during inpatient visits, an average of 7 and 3.5 hours are required for new patient and follow-up patient-related activities, respectively [16], which comprise a greater workload than any other specialty [17].
In addition to the workload, GC practitioners require certain training and certifications in order to address the medical, psychological, and familial implications of patients with genetic diseases [18]. In view of clinical practice, medical roles, psychosocial support, and case management practices are mostly needed [19]. However, such roles may not be sufficiently provided by one person, as management of genetic conditions can vary and is difficult to generalize [2021]. Integrating genomic information in medical curricula may help in providing genetic knowledge to the physicians enabling better communication. A flexible rather than a uniform GC model may be more adequate realistically; however, this requires effective communication between specialty physicians, as well as transforming genomic information into clinical laboratory results. Laboratory physicians are proficient at medical communication and play an essential role in translating genetic findings into clinical reports [22].
Given the uniform national healthcare insurance policy and legal requirements for medical personnel in Korea, establishing a GC service within the Korean National Health Insurance System (KNHIS) presents several difficulties. The main reason for the shortage of GC providers is lack of financial support, which is mainly influenced by the KNHIS. From a cost perspective, pre-testing GC can guide suitable genetic testing, which can minimize unnecessary testings, and post-testing GC can offer precise disease management for specific diseases [23]. The benefits of providing qualified GC either in a patient-specific manner or as a public health measure are invaluable. To secure qualified GC service in Korea, the KNHIS policy regarding reimbursement for GC should be amended.
As noted in our survey, 23.1% of laboratory physicians are currently providing GC services regardless of the current challenges, and some are preparing to establish new GC clinics. This finding reflects increasing demand for genetic testing, especially using the NGS panel. Where GC cannot be directly provided by genetic counselors, general physicians may perform GC, especially in oncology clinics. As adoption of the knowledge and skills required to perform specific genetic tasks takes years and the annual growth rate for GC providers in Korea is far lower than in other countries [1213], providing GC services by laboratory physicians or clinicians familiar with genetics seems to be a reasonable alternative solution at this time.
ACKNOWLEDGEMENTS
We acknowledge the members of Korean Society of Laboratory Medicine for participating in this survey and staff of Korean Society of Genetic Diagnostics who compiled data for this study.
Notes
AUTHOR CONTRIBUTIONS: Jieun Kim participated in summarizing data and writing of the paper. Sun-Young Kong participated in research design and advised on research methodology. Sung-Hee Han participated in research design and advised on research methodology. Jong-Won Kim participated in critically revising the study and provided support. Chang Ho Jeon participated in critically revising the study and provided support. Jongha Yoo participated in the performance of the research and supervised the full study.
References
1. Leopold JA, Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018; 122:1302–1315. PMID: 29700074.
2. Lee S, Choi M. Ultra-rare disease and genomics-driven precision medicine. Genomics Inform. 2016; 14:42–45. PMID: 27445646.
3. Sohn J. Next generation sequencing and anti-cancer therapy. J Korean Med Assoc. 2019; 62:119–129.
4. Balmaña J, Sanz J, Bonfill X, Casado A, Rué M, Gich I, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004; 112:647–652. PMID: 15382046.
5. Greeley SAW, John PM, Winn AN, Ornelas J, Lipton RB, Philipson LH, et al. The cost-effectiveness of personalized genetic medicine: the case of genetic testing in neonatal diabetes. Diabetes Care. 2011; 34:622–627. PMID: 21273495.
6. Lawrence WF, Peshkin BN, Liang W, Isaacs C, Lerman C, Mandelblatt JS. Cost of genetic counseling and testing for BRCA1 and BRCA2 breast cancer susceptibility mutations. Cancer Epidemiol Biomarkers Prev. 2001; 10:475–481. PMID: 11352857.
7. Arora NS, Davis JK, Kirby C, McGuire AL, Green RC, Blumenthal-Barby JS, et al. Communication challenges for nongeneticist physicians relaying clinical genomic results. Per Med. 2016; 14:423–431. PMID: 29181085.
8. Kim HJ. Genetic counseling in Korean health care system. J Genet Med. 2011; 8:89–99.
9. Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015; 17:169–176. PMID: 25210938.
10. Lee YW, Ki CS, Shin HB, Choi TY, Kim JW, Kim SH, et al. Establishment and management of medical genetic clinic based on genetic testing network. Korean J Lab Med. 2006; 26:137–141. PMID: 18156715.
11. Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014; 160:255–266. PMID: 24366442.
12. Laurino MY, Leppig KA, Abad PJ, Cham B, Chu YWY, Kejriwal S, et al. A report on ten Asia Pacific countries on current status and future directions of the genetic counseling profession: the establishment of the Professional Society of Genetic Counselors in Asia. J Genet Couns. 2018; 27:21–32. PMID: 28699126.
13. Hoskovec JM, Bennett RL, Carey ME, DaVanzo JE, Dougherty M, Hahn SE, et al. Projecting the supply and demand for certified genetic counselors: a workforce study. J Genet Couns. 2018; 27:16–20. PMID: 29052810.
14. Kausmeyer DT, Lengerich EJ, Kluhsman BC, Morrone D, Harper GR, Baker MJ. A survey of patients’ experiences with the cancer genetic counseling process: recommendations for cancer genetics programs. J Genet Couns. 2006; 15:409–431. PMID: 17106634.
15. Lee CH, Lim H, Kim Y, Yoon S, Park YS, Kim SA, et al. Analysis of new patient’s willingness to pay additional costs for securing satisfactory consultation time. Health Policy Manag. 2017; 27:39–46.
16. McPherson E, Zaleski C, Benishek K, McCarty CA, Giampietro PF, Reynolds K, et al. Clinical genetics provider real-time workflow study. Genet Med. 2008; 10:699–706. PMID: 18978682.
17. Heald B, Rybicki L, Clements D, Marquard J, Mester J, Noss R, et al. Assessment of clinical workload for general and specialty genetic counsellors at an academic medical center: a tool for evaluating genetic counselling practices. NPJ Genom Med. 2016; 1:16010. PMID: 29263811.
18. National Society of Genetic Counselors' Definition Task Force. Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, et al. A new definition of genetic counseling: National Society of Genetic Counselors' task force report. J Genet Couns. 2006; 15:77–83. PMID: 16761103.
19. Ormond KE, Laurino MY, Barlow-Stewart K, Wessels TM, Macaulay S, Austin J, et al. Genetic counseling globally: where are we now? Am J Med Genet C Semin Med Genet. 2018; 178:98–107. PMID: 29575600.
20. Zatz M, Passos-Bueno MR, Vainzof M. Neuromuscular disorders: genes, genetic counseling and therapeutic trials. Genet Mol Biol. 2016; 39:339–348. PMID: 27575431.
21. Caso R, Beamer M, Lofthus AD, Sosin M. Integrating surgery and genetic testing for the modern surgeon. Ann Transl Med. 2017; 5:399. PMID: 29152499.
22. Lehr HA, Bosman FT. Communication skills in diagnostic pathology. Virchows Arch. 2016; 468:61–67. PMID: 26481246.
23. Agnese DM, Pollock RE. Breast cancer genetic counseling: a surgeon's perspective. Front Surg. 2016; 3:4. PMID: 26858951.
Table 1
Summary of the characteristics of the 52 survey respondents
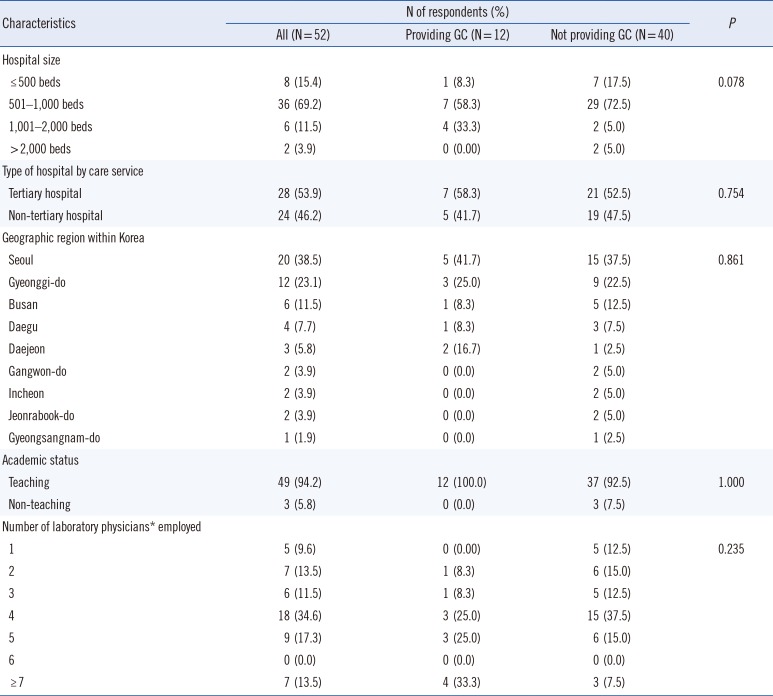
Table 2
Status of the 12 respondents providing GC
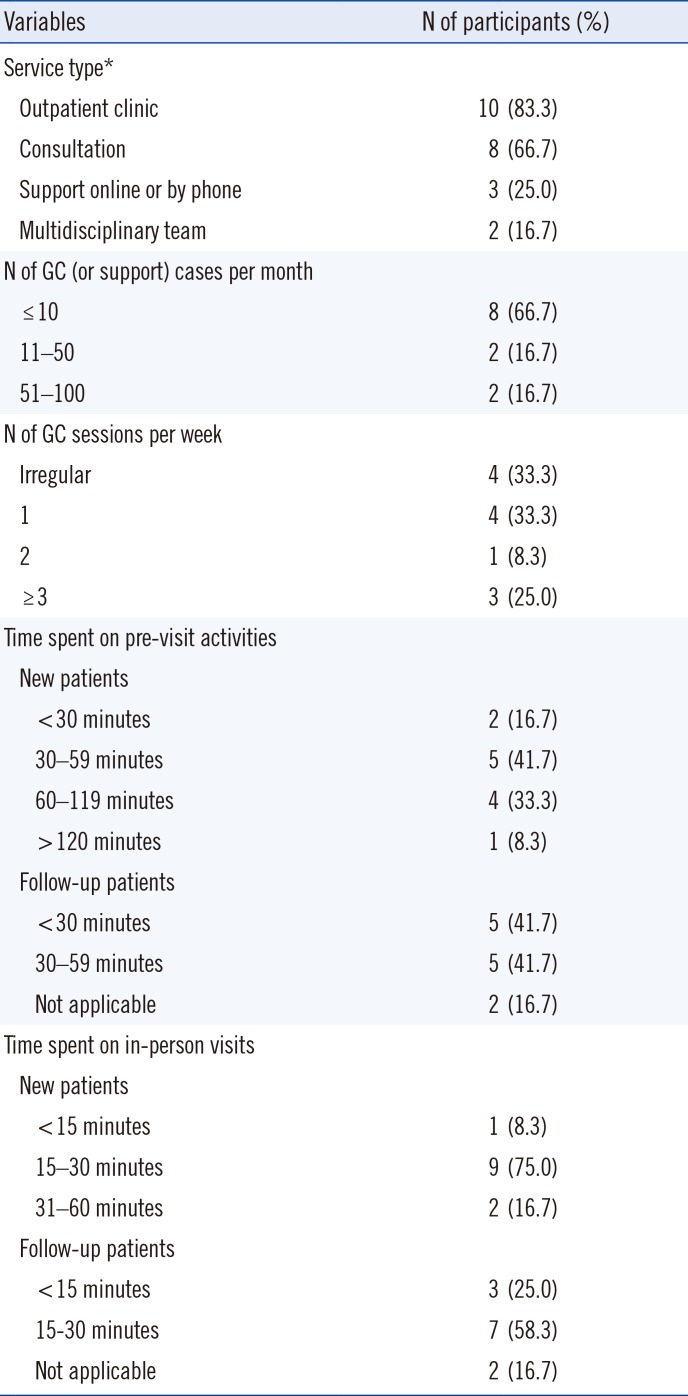
Table 3
Concerns and difficulties (or issues) in performing GC as a laboratory physician




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download