Abstract
Background
Methods
Results
Conclusions
Notes
AUTHOR CONTRIBUTIONS: JHB and DY wrote the manuscript. JYC and YSP aided sample collection and interpreting the results. JH B, DY, and YAK planned and carried out the simulations. MK and BK conceived and carried out the experiments. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
FUNDING: This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries through the Agricultural Microbiome R&D Program, funded by the Ministry of Agriculture, Food and Rural Affairs (918003-4); by the Korea Health Technology R&D Project through the KHIDI funded by the Ministry of Health & Welfare, Korea (grant number HI17C180,7); and by the BioNano Health Guard Research Center funded by the MSIP of Korea as a Global Frontier Project (H-GUARD_2014M3A6B2060509). This study was also partly supported by Cepheid. The funders had no role in study design, data collection, and interpretation, or in the decision to submit the work for publication.
References
Fig. 2
Comparison of number, median Ct value and interquartile range in (A) the concordant group at initial assay (25.4, 22.2–30.9), resolved discrepancy group after discrepant analysis (28.5, 22.3–31.7), and discordant groups (52, 35.0, 31.4–37.7) and (B) the concordant (24.4, 22.2–30.1) and discordant (35.5, 29.9–36.0) KPC groups and concordant (32.2, 27.5–34.6) and discordant (34.8, 31.3–36.5) NDM groups.
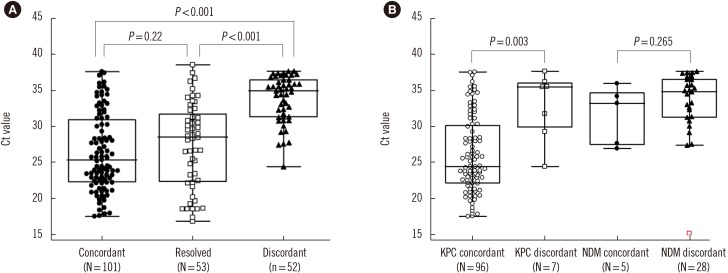
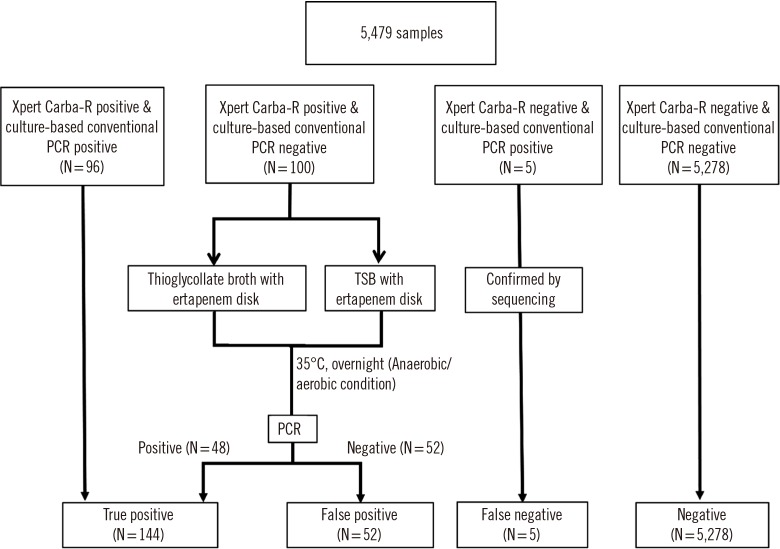
Table 1
Comparison of overall performance of the Xpert Carba-R v.2 and culture-based PCR method
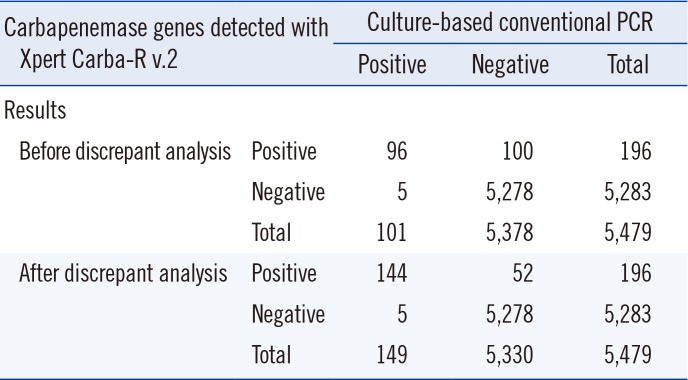
Table 2
Sensitivity, specificity, PPV, and NPV according to carbapenemase type with discrepant analysis
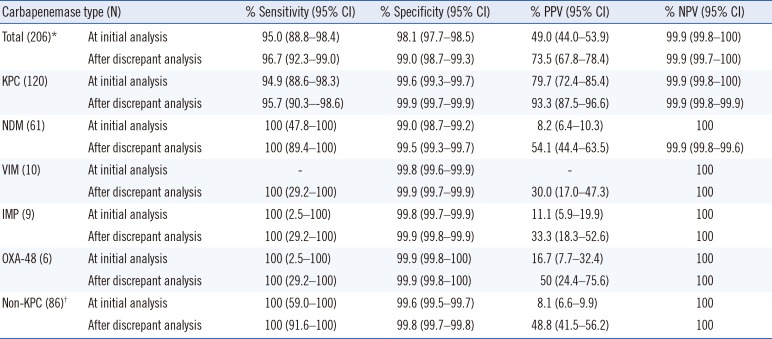
*The number of positive samples was counted; samples harboring multiple genes were counted as one sample.
†Non-KPC includes NDM, VIM, IMP, and OXA-48.
Abbreviations: CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; VIM, Verona integron-mediated metallo-β-lactamase; IMP, imipenem; OXA-48, oxacillinase-48.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download