Abstract
Purpose
To evaluate the in vitro impact of ritalinic acid (RA), a major metabolite of methylphenidate (drug to treat attention-deficit hyperactivity disorder), on sperm motility, vitality and oxidative stress.
Materials and Methods
Semen samples (n=13) were collected from healthy donors and a semen analysis was performed according to World Health Organization. Density gradient centrifugation was performed to isolate motile sperm. Samples were incubated with different concentrations (0, 1, 10, 100, and 1,000 ng/mL) of RA. The non-exposed group (0 ng/mL) was defined as the control group. Samples were analyzed for motility at different time points (0, 60, 150, 240, and 300 minutes) and for vitality and oxidation reduction potential (ORP) (at 0, 240, and 300 minutes). Sperm motility was assessed manually and motion kinetic parameters were recorded by computer aided semen analysis.
Results
RA at any tested concentration significantly increased sperm motility compared to the control in a time-dependent manner with a maximum increase after 240 minutes. Motion kinetic parameters were not comparable. For sperm vitality, supplementation with RA significantly maintained survival at higher levels, while non-treated sperm gradually died. These higher levels of vitality were maintained with rising RA concentrations of up to 1,000 ng/mL. A non-significant trend of increased ORP was observed in all study groups.
Attention-deficit hyperactivity disorder (ADHD) is a frequent neurodevelopmental disorder with an increasing prevalence over the last 2 decades [1]. A recent cohort study of almost 7.5 million people with roughly 700,000 prescriptions, reported an increase in prescription rate from 42.7 prescriptions per 10,000 persons before the year 2000 to 394.4 prescriptions in 2015 an increase of about 800% in prescription rate [2]. The upsurge was observed in both sexes and in all age groups. Moreover, unlike previous trends of increased diagnosis and treatment of ADHD in pre-school and school aged children, the later increase applies to children, adolescents, and even adults [3].
The most commonly used first line pharmacologic treatment for ADHD is methylphenidate (MPH). The mechanism of action of MPH in ADHD is still a matter of debate, but several studies indicated that MPH inhibits the presynaptic dopamine transporter [45]. The mechanism of action includes inhibition of the re-uptake of norepinephrine and dopamine into the presynaptic neuron or an increase of their intrasynaptic concentrations by increasing their release into the extraneuronal space [6].
Ritalinic acid (RA), the major inactive metabolite of MPH, is one of the isomers of amphetamine. The circulating concentrations of RA greatly exceed that of the parent drug [7]. If MPH is given in the normal dosage for clinical use, the plasma concentration of RA reaches a maximal concentration of 10 ng/mL with a half-life of 2.5 hours [8]. RA is soluble in water and due to its small size, it readily passes the blood brain and testicular barriers [9].
Due to the wide use of MPH to treat ADHD, several studies have been conducted to evaluate its impact on various body biological systems in general and the reproductive system in particular; however, most of them are animal studies. Chronic use of MPH decreases the body weights of male mice as compared to a control group. In addition, the weight of the testes and seminal vesicles decreased significantly [10]. MPH was reported to reduce the number of Leydig cells and serum testosterone levels, increase the percentage of abnormal tail and sperm cell morphology and the testicular interstitial tissue volume, along with a reduction in fertility rates of the male mice in the MPH treated groups compared to controls [1112]. MPH was reported to negatively affect spermatogenesis not only by reducing testicular weight and number of round spermatids but also by increasing apoptotic death and p53 activation [13].
Previous studies, mainly animal ones, investigated the impact of orally administrated MPH on biological systems. However, no study assessed the effect of MPH or its metabolites on human sperm. This lack of information might be related to the young age of the patients, mostly prepubertal boys, to whom MPH was prescribed at the time the drug was approved for clinical use in 1955. Nonetheless, the growing use of MPH in adolescents and adults who are in their reproductive age, and the growing rate of MPH abuse, requires an evaluation of the impact of MPH on male reproduction. The aim of the present study is to investigate the direct effect of RA on human semen parameters in vitro.
Following the approval of the study by the Institutional Review Board (IRB) of the Cleveland Clinic Foundation Hospital (Reg. No. 15-435) according to the Declaration of Helsinki, a total of 13 semen samples were collected from healthy donors by masturbation after 2 to 3 days of abstinence. An informed, written consent has been obtained from each donor.
All samples were allowed to liquefy completely for 20 minutes at 37℃ before further processing. The initial semen analysis was performed according to the World Health Organization (WHO) 5th edition [14]. Density gradient centrifugation was performed to isolate motile sperm. Given the achieved plasma concentration of 10 ng/mL during treatment, each sample was incubated with different concentrations of RA (1, 10, 100, and 1,000 ng/mL RA) and compared to a control without RA from the same patient. Depending on the volume of semen available, not all tests could be performed for each donor. However, a minimum of 8 samples was used.
The following inclusion criteria were applied for samples to be eligible for enrollment into the study: generally healthy males over 18 years of age who were willing to abstain from sexual activities for 2 to 3 days prior to semen collection, and normal semen analysis as per WHO 2010 guidelines. Subjects presenting with any of the following were excluded: sample of high viscosity semen, non-compliance with protocol requirements, and any abnormal semen parameter according to the WHO 2010 criteria.
Following liquefaction, after the sample has been in the incubator for 20 minutes at 37℃, the sample is examined for viscosity by the ease of pipetting in a graduated serological pipette. Viscosity is graded as follows: Normal viscosity: the sample can easily pass in the pipette and expelled easily, slight viscosity: the sample is not expelled easily out of the pipette, moderate: difficult to pipette and expel, high viscosity: the sample is a coagulum and cannot be aspirated. In the current study, none of our donors presented with moderate or high viscosity. Hence none of the samples were excluded. Semen specimens were evaluated for volume, liquefaction, color, pH, and volume as well as sperm concentration, motility, and vitality as per WHO 2010 guidelines. Motile sperm were obtained by pooling of semen samples prepared by double density gradient separation. The motile sperm were tested for the effect of different concentrations of RA (1, 10, 100, and 1,000 ng/mL) on spermatozoa.
In order to evaluate the better fraction of motile sperm, spermatozoa were prepared by separation on a double density gradient by layering 2 mL each of upper (45%) and lower phase (90%) layer (Vitrolife, San Diego, CA, USA). Two mL of liquefied semen were layered on top. Samples were centrifuged at 300 ×g for 20 minutes at room temperature. The resulting pellet was aspirated and re-suspended with 2 mL of human tubal fluid (HTF) medium supplemented with 2 mg/mL human serum albumin. The final concentration was adjusted to 20×106 motile spermatozoa/mL and 0.5 mL aliquots were used for sperm motility studies.
A human sperm motility assay was used to test the toxicity of the RA at different concentrations [15]. We used a computer assisted semen analyzer IVOS II, ver. 14 (Hamilton Thorne, Beverly, MA, USA). The setting included a heated stage set at 37℃. Spermatozoa in HTF suspensions was exposed to RA at increasing concentrations (1, 10, 100, and 1,000 ng/mL) at 37℃ in an incubator in an atmosphere with 5% CO2 for 1, 2.5, 4, and 5 hours. After incubation, samples were vigorously mixed by pipetting and an aliquot of the test sample was placed on a MicroCell chamber and examined under phase contrast at ×20 magnification for motility. Motion kinetic parameters (curvilinear velocity, VCL; amplitude of lateral head displacement, ALH; linearity, LIN) were recorded by computer aided semen analysis (CASA, IVOS II; Hamilton Thorne).
Sperm vitality was tested using the eosin-nigrosine stain. An amount of 0.67 g eosin Y (color index 45,380) and 0.9 g sodium chloride (NaCl) were dissolved in 100 mL of purified water. Ten grams of nigrosin (color index 50,420) were added to the 100 mL of eosin Y solution. Semen samples were mixed with an equal volume of eosin-nigrosin suspension in a porcelain spot plate well or test-tube. A smear on a glass slide was made from each suspension and was allowed to air dry. Immediately after drying, each slide was examined at ×1,000 magnification under oil immersion. The number of stained (dead) or unstained (vital) cells was evaluated and the percentage of vital sperm was calculated.
Oxidation reduction potential (ORP) levels were measured in sperm suspension by using the MiOXSYS analyzer (Aytu Bioscience, Eaglewood, CO, USA) at 0 and 240 and 300 minutes parallel to the vitality and motility tests. ORP measures the transfer of electrons from a reductant (or antioxidant) to an oxidant. Briefly, 30 mL of liquefied semen at room temperature were applied to the MiOXSYS sensor. The sensor was pre-inserted into the MiOXSYS analyzer; measurements begin automatically. ORP is measured in milli-volts (mV) and is the integrated measure of the existing balance between total oxidants and reductants in a biological system.
Each sample was measured in triplicate, and the average values for ORP were recorded. Data were then normalized to sperm concentration to control for differences in cell numbers. Thus, data are presented as mV/106 sperm/mL. To assess the effects of time on ORP measures, samples were incubated at room temperature for 0 and 120 minutes before being measured.
For statistical analysis, MedCalc Statistical Software, ver. 17.4 (MedCalc Software bvba, Ostend, Belgium) was used. After testing for normal distribution of the data by means of the Kolmogorov—Smirnov test, the parametric (paired-samples t-test) and non-parametric tests (Wilcoxon test and Kruskal-Wallis test with Jonckheere-Terpstra trend), respectively, were employed. Data are presented as mean±standard error of mean. A p<0.05 was considered significant.
All semen samples underwent an initial semen analysis according to the WHO 5th edition. All samples were found eligible and no sample was required to be excluded.
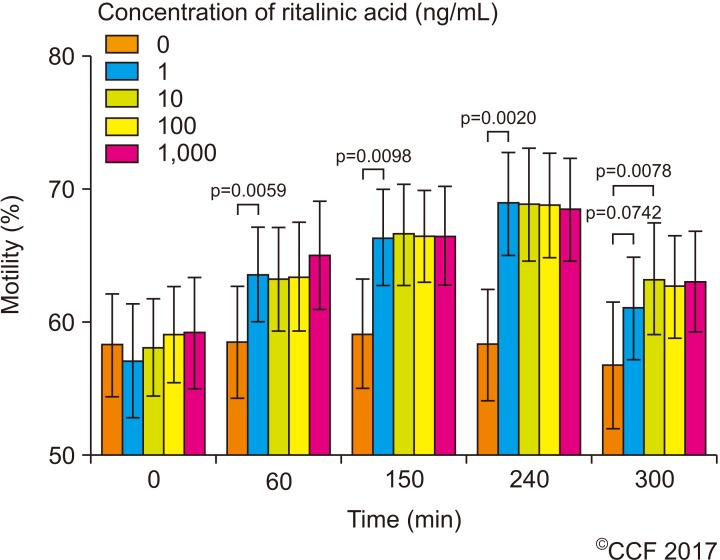
A total of 10 samples were evaluated for sperm motility. Samples were analyzed at different time points (0, 60, 150, 240, and 300 minutes) based on the half-life of RA in plasma. All concentrations of RA used in this study significantly (p<0.05) increased sperm motility with a maximum after 240 minutes of incubation (Fig. 1). This increase was maintained with rising concentrations of up to 1,000 ng/mL. Thereafter, a decrease was observed at 300 minutes of incubation in treated samples, even though motility remained significantly higher compared to control. From the control to the 240 minutes incubation point, the Jonckheere-Terpstra trend test indicated for all concentrations significant trends (p<0.05), though the Kruskal-Wallis test was not significant (p=0.2473 to p=0.1315). No significant changes were seen in sperm motion kinetic parameters.
A total of 13 samples were evaluated for sperm vitality (Fig. 2; Supplementary Fig. 1). Since an increase in sperm motility was observed in 60 and 150 minutes, vitality would not be decreased at these time points, sperm vitality was instead analyzed at 3 time points (0, 240, and 300 minutes). A reduction in vitality was observed as compared to the control group without addition of RA. The addition of RA to the sperm samples caused sperm to retain their vitality.
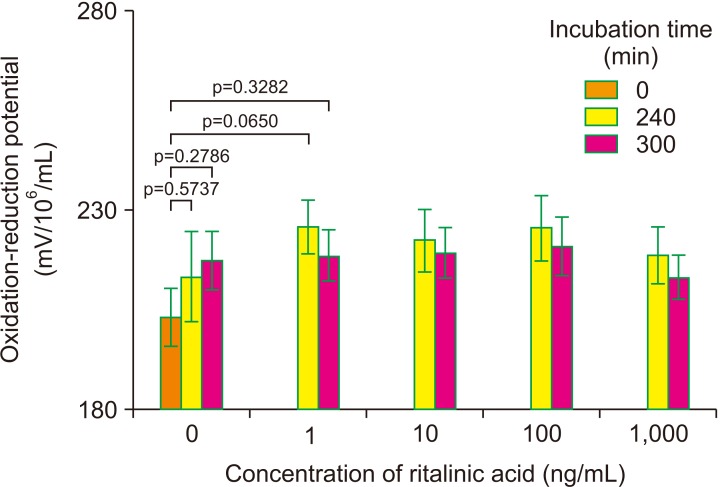
A total of 8 samples were evaluated for ORP before and after exposure to RA. Samples were analyzed at 3 time points (0, 240, and 300 minutes) after the observed increase in sperm motility was observed in 60 and 150 minutes (Fig. 3; Supplementary Fig. 2). A nonsignificant trend (p=0.3125 and p=0.1953) of increase in ORP levels compared to the control was observed in all study groups after 240 and 300 minutes of incubation, respectively.
MPH in its various administration forms is widely used for treatment of ADHD in the general population, including those patients at reproductive age. Increasing age of the treated patients and increased rates of MPH abuse have been reported as well [16]. The direct impact of MPH on human sperm and the resulting impact on male reproduction is still unknown. This study is the first indicating that MPH metabolite, RA, has a temporary effect on sperm function including increased vitality, motility and possibly increased reactive oxygen species (ROS) levels as compared to controls.
Results of the current study clearly show that MPH main metabolite, RA, was able to increase human sperm motility, while sperm kinematic parameters, such as LIN, ALH, and VCL were not affected. In addition, vitality was maintained over time at significantly higher levels as compared to the control. It is also important to note that despite increased oxidative stress (OS) levels as measured as ORP after 240 and 300 minutes, respectively, these increases were not significant. Moreover, the impact of RA on sperm functions was comparable in all studied RA concentrations including the subclinical dose of 1 ng/mL, and the abuse equivalent doses of 100 and 1,000 ng/mL. However, due to the fact that the actual RA concentrations in semen have never been reported before, concentrations used in this study were only based on plasma levels and has to be considered as a limitation of our study.
The exact mechanism of action of MPH in ADHD treatment is still debatable. It is hypothesized that MPH acts as a catecholamine reuptake inhibitor impacting mainly dopamine and norepinephrine levels. MPH blocks the dopamine transporter in the central nervous system, thus causing an increased dopamine concentration in the synaptic cleft [17]. In the male reproductive system, high concentrations of catecholamines including dopamine [18] and the presence of dopamine receptors in the sperm cell [19] including a2- and b-adrenergic receptors and the dopamine type 2 receptor in testis, as well as spermatogenic cells and sperm cells have been described [20]. Catecholamines were reported to impact sperm cell motility [21]. While evaluating the impact of dopamine on sperm motility, Urra et al [19] exposed human sperm to different dopamine concentrations. An inhibition of total sperm motility and progressive motility was observed after incubation for 1, 3, and 6 hours at a dopamine concentration of 1 mM. Nevertheless, exposing sperm to dopamine in the absence and presence of a specific dopamine transporter inhibitor, the dopamine-induced inhibition of total sperm motility was partially reversed. Thus, the temporary increase in sperm motility observed in the current study corresponding to the MPH half-life may be explained by the inhibition of the dopamine uptake inhibition.
The observed increased motility cannot be attributed to decreased OS levels as measured by ORP since these slightly, but not significantly, increased. At this stage, it can also only be speculated whether this increased ROS activity is due to increased metabolic activity of the spermatozoa or simply caused by the elimination of protective antioxidants present in the seminal plasma. Excessive ROS production that impairs the fine redox balance between oxidants and anti-oxidants (enzymatic and non-enzymatic) yield OS. In turn, OS has a well-established deteriorating effect on sperm function including reduced sperm motility, decreased ability of spermatozoa to adhere to the oocyte [22], to undergo capacitation [23] as well as OS-induced DNA damage, mainly DNA fragmentation [24]. Previous studies reported that a large percentage of male factor infertility is due to OS-mediated sperm damage [2526]. In addition, several extrinsic factors such as smoking [2728] or varicocele [29] may cause OS.
On the other hand, naturally generated low ROS levels are major role players in male reproductive physiologic processes such as spermatogenesis and the triggering of sperm capacitation. Ultimately, the molecular processes involved in sperm binding to the zona pellucida are also mediated by hydrogen peroxide. Likewise, ROS in low levels are important for proper sperm motility [24]. ROS, in general, are essential for proper spermatogenic mechanisms to occur, and hydrogen peroxide, in particular, stimulates acrosomal reaction, tyrosine phosphorylation and hyperactivation in the male germ cell. The latter, in turn is a direct expression of changes taking place in the sperm kinematic parameters VCL (>150 µm/s), LIN (<50%), and ALH (>7 µm). Considering that these parameters did not change during the incubation with MPH, however, one can assume that MPH did not stimulate intrinsic ROS production which, in turn, would trigger capacitation, sperm hyperactivation and acrosome reaction. On the other hand, sperm exposed to dopamine and norepinephrine after facilitating capacitation with heparin were reported to have a dose-dependent effect on the acrosome integrity [1920] implying that sperm would not be able to fertilize the oocyte by the time it will reach it as capacitation and/or the acrosome reaction are prematurely triggered.
A potential application of the observed impact of RA (or any other dopamine reuptake inhibitor) on sperm cells may be as a motility enhancer. This would be of particular interest in patients with asthenozoospermia or during various fertility treatment procedures such as intra-uterine insemination (IUI). IUI is often a first line treatment in patients with adequate sperm numbers since it is minimally invasive and far less expensive when compared to in-vitro fertilization. It is used when there is only mildly reduced sperm count, motility or morphology as well as in cases of unexplained infertility. In this procedure, the sperm avoids the hostile vaginal environment and the need to pass through the uterine cervix. However, the transport to the fallopian tube and more importantly fertilization of the egg remains. Hence, an increased sperm motility observed after the use of RA may be used to increase motility and therefore potentially increase the effectiveness of IUI. However, before using RA during fertility treatments, its impact on sperm capacitation, acrosome reaction and sperm DNA must be determined. These processes are major role players in the fertilizing competence of sperm and are essential to enable the sperm to penetrate the oocyte and cause fertilization [30].
The current study has some limitations. The first is the small study sample of the evaluated parameters. Another limitation is the fact that only selected sperm parameters including vitality, total motility, VCL, ALH, LIN, and ORP were measured. However, the fact that despite the small sample significant differences were observed between the study groups reflects the substantial effect of RA on sperm function parameters.
In conclusion, RA significantly increased sperm motility and maintained vitality over a long incubation period. This may allow its in vitro use to improve sperm motility in cases of patients with asthenozoospermia. However, it is unknown what effects RA has on other sperm functional characteristics such as: capacitation, acrosome reaction, intrinsic ROS production and sperm nuclear DNA fragmentation. Further investigation is required prior to considering its clinical use.
ACKNOWLEDGEMENTS
The study was supported by funds from the American Center for Reproductive Medicine. Andrology lab technologists helped with scheduling of subjects. Figures were drawn by graphic artists from the Cleveland Clinic Arts department.
Special thanks to: Reva Prabha Peer, Ibrahim Altamimi, Mohamed Iesar Mohamed, Muna Abuayash, Abdullah Alzaaqi, Fares Bamajbuor.
Notes
References
1. Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012; 130:23–31. PMID: 22711728.

2. Renoux C, Shin JY, Dell'Aniello S, Fergusson E, Suissa S. Prescribing trends of attention-deficit hyperactivity disorder (ADHD) medications in UK primary care, 1995–2015. Br J Clin Pharmacol. 2016; 82:858–868. PMID: 27145886.

3. Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am J Psychiatry. 2015; 172:967–977. PMID: 25998281.

4. Jenson D, Yang K, Acevedo-Rodriguez A, Levine A, Broussard JI, Tang J, et al. Dopamine and norepinephrine receptors participate in methylphenidate enhancement of in vivo hippocampal synaptic plasticity. Neuropharmacology. 2015; 90:23–32. PMID: 25445492.
5. Kodama T, Kojima T, Honda Y, Hosokawa T, Tsutsui KI, Watanabe M. Oral administration of methylphenidate (ritalin) affects dopamine release differentially between the prefrontal cortex and striatum: a microdialysis study in the monkey. J Neurosci. 2017; 37:2387–2394. PMID: 28154152.

6. Volkow ND, Wang GJ, Smith L, Fowler JS, Telang F, Logan J, et al. Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. Neuroimage. 2015; 121:20–28. PMID: 26208874.

7. Modi NB, Wang B, Noveck RJ, Gupta SK. Dose-proportional and stereospecific pharmacokinetics of methylphenidate delivered using an osmotic, controlled-release oral delivery system. J Clin Pharmacol. 2000; 40:1141–1149. PMID: 11028253.
8. Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, et al. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983; 226:382–386. PMID: 6410043.
9. Adjei A, Teuscher NS, Kupper RJ, Chang WW, Greenhill L, Newcorn JH, et al. Single-dose pharmacokinetics of methylphenidate extended-release multiple layer beads administered as intact capsule or sprinkles versus methylphenidate immediate-release tablets (Ritalin(®)) in healthy adult volunteers. J Child Adolesc Psychopharmacol. 2014; 24:570–578. PMID: 25514542.

10. Beckman DA, Schneider M, Youreneff M, Tse FL. Juvenile toxicity assessment of d,l-methylphenidate in rats. Birth Defects Res B Dev Reprod Toxicol. 2008; 83:48–67. PMID: 18189274.

11. Fazelipour S, Jahromy MH, Tootian Z, Kiaei SB, Sheibani MT, Talaee N. The effect of chronic administration of methylphenidate on morphometric parameters of testes and fertility in male mice. J Reprod Infertil. 2012; 13:232–236. PMID: 23926551.
12. Nudmamud-Thanoi S, Thanoi S. Methamphetamine induces abnormal sperm morphology, low sperm concentration and apoptosis in the testis of male rats. Andrologia. 2011; 43:278–282. PMID: 21486410.

13. Cansu A, Ekinci O, Ekinci O, Serdaroglu A, Erdogan D, Coskun ZK, et al. Methylphenidate has dose-dependent negative effects on rat spermatogenesis: decreased round spermatids and testicular weight and increased p53 expression and apoptosis. Hum Exp Toxicol. 2011; 30:1592–1600. PMID: 21183565.

14. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization;2010.
15. Claassens OE, Wehr JB, Harrison KL. Optimizing sensitivity of the human sperm motility assay for embryo toxicity testing. Hum Reprod. 2000; 15:1586–1591. PMID: 10875871.

16. Cairns R, Daniels B, Wood DA, Brett J. ADHD medication overdose and misuse: the NSW Poisons Information Centre experience, 2004–2014. Med J Aust. 2016; 204:154. PMID: 26937669.

17. Gronier B. In vivo electrophysiological effects of methylphenidate in the prefrontal cortex: involvement of dopamine D1 and alpha 2 adrenergic receptors. Eur Neuropsychopharmacol. 2011; 21:192–204. PMID: 21146374.

18. Fait G, Vered Y, Yogev L, Gamzu R, Lessing JB, Paz G, et al. High levels of catecholamines in human semen: a preliminary study. Andrologia. 2001; 33:347–350. PMID: 11736795.

19. Urra JA, Villaroel-Espíndola F, Covarrubias AA, Rodríguez-Gil JE, Ramírez-Reveco A, Concha II. Presence and function of dopamine transporter (DAT) in stallion sperm: dopamine modulates sperm motility and acrosomal integrity. PLoS One. 2014; 9:e112834. PMID: 25402186.

20. Ramírez AR, Castro MA, Angulo C, Ramió L, Rivera MM, Torres M, et al. The presence and function of dopamine type 2 receptors in boar sperm: a possible role for dopamine in viability, capacitation, and modulation of sperm motility. Biol Reprod. 2009; 80:753–761. PMID: 19074002.
21. Bavister BD, Chen AF, Fu PC. Catecholamine requirement for hamster sperm motility in vitro. J Reprod Fertil. J Reprod Fertil. 1979; 56:507–513. PMID: 480308.
22. Bromfield EG, Aitken RJ, Anderson AL, McLaughlin EA, Nixon B. The impact of oxidative stress on chaperone-mediated human sperm-egg interaction. Hum Reprod. 2015; 30:2597–2613. PMID: 26345691.

23. Morielli T, O'Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction. 2015; 149:113–123. PMID: 25385721.

24. Agarwal A, Durairajanayagam D, Halabi J, Peng J, Vazquez-Levin M. Proteomics, oxidative stress and male infertility. Reprod Biomed Online. 2014; 29:32–58. PMID: 24813754.

25. Shekarriz M, Thomas AJ Jr, Agarwal A. Incidence and level of seminal reactive oxygen species in normal men. Urology. 1995; 45:103–107. PMID: 7817460.

26. Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008; 14:243–258. PMID: 18281241.

27. Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016; 70:635–645. PMID: 27113031.

28. Harlev A, Agarwal A, Gunes SO, Shetty A, du Plessis SS. Smoking and male infertility: an evidence-based review. World J Mens Health. 2015; 33:143–160. PMID: 26770934.

29. Agarwal A, Sharma R, Harlev A, Esteves SC. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl. 2016; 18:163–170. PMID: 26780872.

30. Stival C, Puga Molina Ldel C, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol. 2016; 220:93–106. PMID: 27194351.

Supplementary Materials
Supplementary materials can be found via
https://doi.org/10.5534/wjmh.180127.
Supplementary Fig. 2
The impact of ritalinic acid on oxidation reduction potential.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download