This article has been
cited by other articles in ScienceCentral.
Abstract
We had six cases of patients who were treated with long-term testosterone replacement therapy (TRT) after high dose-rate (HDR) brachytherapy and androgen deprivation therapy for high-risk prostate cancer. All patients were given testosterone enanthate by intramuscular injection every 3 to 4 weeks. Blood biochemistry including prostate specific antigen (PSA) level was evaluated every 3 to 6 months after TRT, and radiological imaging was performed every 12 months. All patients had slight increases in PSA within the normal range and not indicative of biochemical recurrence. A sudden increase in PSA was observed in one patient, but it finally decreased. Aging male symptoms scale and various metabolic factors were improved by TRT in all of cases. Although adverse events included polycythemia in one patient, no patients experienced disease recurrence or progression during TRT. Our results suggest TRT for high risk-patients with HDR brachytherapy for prostate cancer may be beneficial and safe.
Go to :

Keywords: Hypogonadism, Prostatic neoplasms, Safety, Testosterone
A decrease of testosterone can result in lowering of motivation and sexual desire, decrease in muscle mass and bone mineral density, metabolic syndrome, and depressive symptoms [
12]. Testosterone replacement therapy (TRT) improves many of the clinical consequences of hypogonadism and helps to maintain the quality of life of hypogonadal men [
1].
On the other hand, TRT is not recommended for patients with a history of prostate cancer. It widely accepted that testosterone promotes prostate cancer growth, and androgen deprivation therapy (ADT) is seen as an effective treatment for prostate cancer. Concerns of TRT for patients with prostate cancer or a history of prostate cancer include disease progression and recurrence. However, recent studies have demonstrated the safety of TRT for patients who have received radical treatment for prostate cancer [
345678], whereas limited information regarding TRT for the patients with high-risk prostate cancer has been currently available. We had six cases of patients who were successfully treated with long-term TRT after high dose-rate (HDR) brachytherapy and ADT for high-risk prostate cancer.
CASE REPORT
1. Our criteria and protocol for testosterone replacement therapy
Patients with no definitive findings of prostate cancer recurrence for >2 years after treatment based on both serum prostate specific antigen (PSA) and radiological imaging were indicated for TRT. Those with total testosterone (TT) concentrations ≤300 ng/dL were included. TRT was given to patients who experienced hypogonadal symptoms such as fatigue, depressive symptoms, and hot flushes caused by testosterone decline. Stage IV patients with definite metastatic lesions before starting prostate cancer therapy, baseline PSA >0.2 ng/mL before starting TRT, receiving radical treatment within the previous 2 years, or with unstable and poor cancer control as determined by the attending physician, were excluded. TRT was interrupted in patients with PSA elevations of 2.0 ng/mL above the nadir, radiological evidence of disease progression, or any adverse events associated with TRT such as polycythemia.
All patients were given testosterone enanthate (TE) (125 mg or 250 mg; Enarmon Depot®; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) by intramuscular injection every 3 to 4 weeks. Blood biochemistry including PSA and TT was evaluated every 3 to 6 months after TRT, and radiological imaging was performed every 12 months. All blood analyses were performed before TRT at each visit.
2. Ethics statement
The protocol and study procedures were approval by Kanazawa University Hospital Institutional Review Board (no. 2018-171 [2934]). In accordance with the guiding principles of the Declaration of Helsinki, all patients gave their informed consent before inclusion after the risks and benefits of therapy were explained.
3. Clinical characteristics and outcomes of six patients
We reported six patients with TRT for hypogonadal symptoms after HDR brachytherapy of high- or very high-risk prostate cancer (
Table 1,
Fig. 1). All had received brachytherapy combined with adjuvant ADT by combined androgen blockade for 2 years, and five had received external beam radiotherapy (EBRT) following brachytherapy.
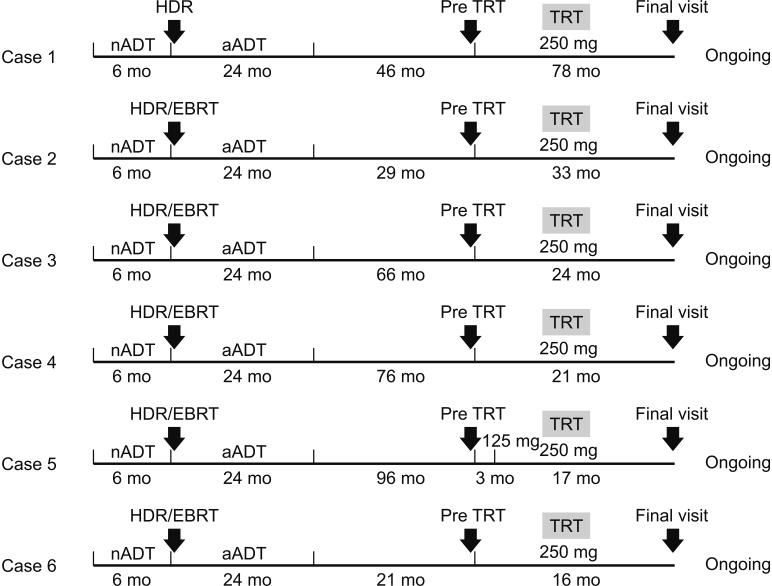 | Fig. 1A flow chart to indicate details in each case, including schedule of therapy, the duration of TRT therapy, follow-up time points and evaluation at each time points. nADT: neoadjuvant androgen deprivation therapy, aADT: adjuvant androgen deprivation therapy, HDR: high dose-rate brachytherapy, TRT: testosterone replacement therapy, EBRT: external beam radiation therapy.
|
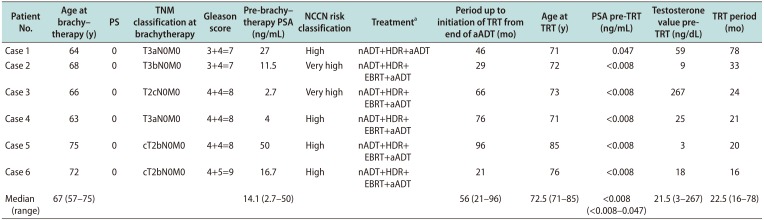
Table 1
Patient characteristics and treatment
|
Patient No. |
Age at brachy-therapy (y) |
PS |
TNM classification at brachytherapy |
Gleason score |
Pre-brachy-therapy PSA (ng/mL) |
NCCN risk classification |
Treatmenta
|
Period up to initiation of TRT from end of aADT (mo) |
Age at TRT (y) |
PSA pre-TRT (ng/mL) |
Testosterone value pre- TRT (ng/dL) |
TRT period (mo) |
|
Case 1 |
64 |
0 |
T3aN0M0 |
3+4=7 |
27 |
High |
nADT+HDR+aADT |
46 |
71 |
0.047 |
59 |
78 |
|
Case 2 |
68 |
0 |
T3bN0M0 |
3+4=7 |
11.5 |
Very high |
nADT+HDR+EBRT+aADT |
29 |
72 |
<0.008 |
9 |
33 |
|
Case 3 |
66 |
0 |
T2cN0M0 |
4+4=8 |
2.7 |
Very high |
nADT+HDR+EBRT+aADT |
66 |
73 |
<0.008 |
267 |
24 |
|
Case 4 |
63 |
0 |
T3aN0M0 |
4+4=8 |
4 |
High |
nADT+HDR+EBRT+aADT |
76 |
71 |
<0.008 |
25 |
21 |
|
Case 5 |
75 |
0 |
cT2bN0M0 |
4+4=8 |
50 |
High |
nADT+HDR+EBRT+aADT |
96 |
85 |
<0.008 |
3 |
20 |
|
Case 6 |
72 |
0 |
cT2bN0M0 |
4+5=9 |
16.7 |
High |
nADT+HDR+EBRT+aADT |
21 |
76 |
<0.008 |
18 |
16 |
|
Median (range) |
67 (57–75) |
|
|
|
14.1 (2.7–50) |
|
|
56 (21–96) |
72.5 (71–85) |
<0.008 (<0.008–0.047) |
21.5 (3–267) |
22.5 (16–78) |

The median age at diagnosis was 67 years. The median initial PSA value was 14.1 ng/mL. Following the 2016 National Comprehensive Cancer Network (NCCN) risk classification guidelines, including initial PSA, tumor stage, and Gleason score (GS), four patients had high-risk, and two had very high-risk prostate cancer. The median period up to initiation of TRT from end of adjuvant ADT was 56 months (range, 21–96 months). The median PSA and TT values at the start of TRT were <0.008 ng/mL (range, <0.008–0.047 ng/mL) and 21.5 ng/dL (range, 3–267 ng/dL), respectively. Five patients had received TE 250 mg per 4 weeks from the beginning, whereas one case (case 5) had 125 mg per 3 weeks for the first 3 months for monitoring adverse effects, and then had 250 mg per 4 weeks. Those patients received TRT for 16 to 78 months.
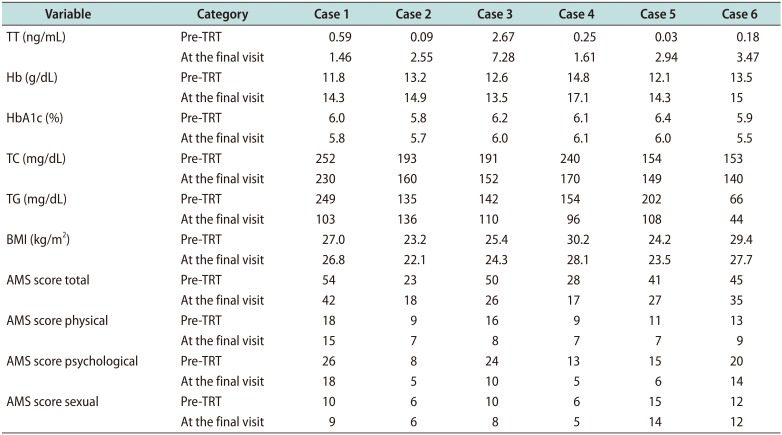
Five patients had slight increases in PSA level that were within the normal range and not indicative of biochemical recurrence. A sudden increase in PSA to 0.843 ng/mL, without any unusual clinical symptoms, was observed in one patient 18 months after starting TRT, but it gradually decreased to 0.338 ng/mL. In most of the cases, hemoglobin (Hb), total cholesterol (TC), triglycerides (TG), hemoglobin A1c (HbA1c), and TT values, and body mass index (BMI) were definitely improved at the final visit after TRT (
Table 2). Aging male symptoms (AMS) score and sub-scales were also mostly improved at follow-up compared with baseline.
Table 2
Patient outcomes at follow-up
|
Variable |
Category |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
Case 6 |
|
TT (ng/mL) |
Pre-TRT |
0.59 |
0.09 |
2.67 |
0.25 |
0.03 |
0.18 |
|
At the final visit |
1.46 |
2.55 |
7.28 |
1.61 |
2.94 |
3.47 |
|
Hb (g/dL) |
Pre-TRT |
11.8 |
13.2 |
12.6 |
14.8 |
12.1 |
13.5 |
|
At the final visit |
14.3 |
14.9 |
13.5 |
17.1 |
14.3 |
15 |
|
HbA1c (%) |
Pre-TRT |
6.0 |
5.8 |
6.2 |
6.1 |
6.4 |
5.9 |
|
At the final visit |
5.8 |
5.7 |
6.0 |
6.1 |
6.0 |
5.5 |
|
TC (mg/dL) |
Pre-TRT |
252 |
193 |
191 |
240 |
154 |
153 |
|
At the final visit |
230 |
160 |
152 |
170 |
149 |
140 |
|
TG (mg/dL) |
Pre-TRT |
249 |
135 |
142 |
154 |
202 |
66 |
|
At the final visit |
103 |
136 |
110 |
96 |
108 |
44 |
|
BMI (kg/m2) |
Pre-TRT |
27.0 |
23.2 |
25.4 |
30.2 |
24.2 |
29.4 |
|
At the final visit |
26.8 |
22.1 |
24.3 |
28.1 |
23.5 |
27.7 |
|
AMS score total |
Pre-TRT |
54 |
23 |
50 |
28 |
41 |
45 |
|
At the final visit |
42 |
18 |
26 |
17 |
27 |
35 |
|
AMS score physical |
Pre-TRT |
18 |
9 |
16 |
9 |
11 |
13 |
|
At the final visit |
15 |
7 |
8 |
7 |
7 |
9 |
|
AMS score psychological |
Pre-TRT |
26 |
8 |
24 |
13 |
15 |
20 |
|
At the final visit |
18 |
5 |
10 |
5 |
6 |
14 |
|
AMS score sexual |
Pre-TRT |
10 |
6 |
10 |
6 |
15 |
12 |
|
At the final visit |
9 |
6 |
8 |
5 |
14 |
12 |

Adverse events associated with TRT included polycythemia in one patient (case 4) after 14 months of treatment that resolved following interruption of TRT for 6 months. No patients experienced disease recurrence or progression during the observation period.
Go to :

DISCUSSION
Some recent studies have demonstrated the safety of TRT for 3 to 5 years for patients who have received radical treatment including prostatectomy or low dose-rate (LDR) brachytherapy, with low to intermediate-risk prostate cancer [
345]. On the other hand, the present study included six patients with high- or very high-risk prostate cancer who were treated with HDR-brachytherapy and ADT. Currently, information regarding safety of TRT for the patients with high-risk prostate cancer has been limited. One previous study including a small number of high-risk prostate cancers demonstrated that TRT for median 4.5 years was safely delivered with no evidence of cancer recurrence or progression in 31 low to high-risk patients treated with LDR brachytherapy [
6]. Another retrospective study that included high-risk prostate cancer patients with GS 8 or higher tumors, positive surgical margins, and positive lymph node metastasis found that four of 26 patients (15.4%) with TRT had cancer recurrence, which was lower than the 53.6% incidence rate (15/28 cases) without TRT. TRT after radical prostatectomy may not contribute to an increased risk for disease recurrence even in patients with high-risk prostate cancer [
7]. A cohort study of patients with low to high-risk prostate cancer treated with brachytherapy or EBRT reported biochemical recurrence six of 98 cases (6.1%) after a median 40.8 months of observation, which was acceptable and comparable with other reports [
8]. These findings suggest that TRT is likely to be safely used following radical therapy for prostate cancer regardless of the prostate cancer risk before treatment.
Published evidence of the clinical benefits of TRT in patients with radical therapy for prostate cancer is also extremely limited. One previous study demonstrated that erectile function could improve with TRT among 20 patients after LDR brachytherapy [
5]. In our case series, AMS scale was improved by TRT in all of the present cases. In addition, TRT was accompanied by improvement in Hb, TC, TG, and HbA1c values, and BMI in most of cases. Especially, prostate cancer patients often experience metabolic syndrome caused by testosterone decrease, and TRT may be effective for the prevention of some lifestyle diseases for them. However, the study included only six participants, and therefore statistical differences could not be analyzed in changes of each parameter. Additional studies including a large number of participants are required to reach a more definite conclusion about the clinical benefits.
Four cases (case 2, 4, 5, and 6) still had castration levels in TT at the start of TRT. Several studies showed that the longer ADT treatment may result in taking more long time for testosterone recovery. Similar to our present cases, one previous study demonstrated that more than 50% of patients who received long-term ADT (36 months) combined with HDR brachytherapy did not experience recovery to normal T levels at 5 years after interruption, and approximately 20% of them still had castration levels [
9].
In addition, TE injection, which is solely approved by Japanese insurance system, is a short-acting testosterone; serum TT level shows a rapid peak a few days after injection, and immediately decreases to baseline level 14 days after injection. Indeed, TT level couldn't reach to the normal range in the final visit after treatment in 4 cases (case 1, 2, 4, and 5). Further efficacy may be expected by more frequent administration of TE, although its safety should be confirmed.
There have been no definite criteria to perform TRT for the prostate cancer patients treated with radical therapy. In particular, period up to initiation of TRT from radical therapy and baseline PSA value before TRT differed widely by previous studies. In previous studies, the exclusion criteria also differed, with patients having PSA values of 0.3 to 0.7 ng/mL after brachytherapy not eligible for TRT [
67]. Since the study population included high-risk cases, the exclusion criteria were strictly set as following; baseline PSA >0.2 ng/mL before starting TRT; receiving radical treatment within the previous 2 years.
Currently, TRT has been regarded as a viable option under well consideration based on the perceived potential benefit of treatment weighed against the limited knowledge of potential risks [
10]. We had six cases with hypogonadism successfully treated by TRT following HDR brachytherapy for high-risk prostate cancer, suggesting that TRT among high-risk prostate cancer patients treated with HDR brachytherapy may be beneficial and safe with no evidence of disease progression. However, there have been no randomized controlled studies to demonstrate efficacy and safety of TRT for the patients treated with radical therapy for prostate cancer. Therefore, further prospective randomized studies including more participants with various prostate cancer risk and more extensive evaluation are required to corroborate the results of this study.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download