1. Donath K, Rohrer MD, Beck-Mannagetta J. A histologic evaluation of a mandibular cross section one year after augmentation with hydroxyapatite particles. Oral Surg Oral Med Oral Pathol. 1987; 63:651–655.

2. Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000; 371:10–27.
3. Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001; 71:354–361.

4. Bohner M, Galea L, Doebelin N. Calcium phosphate bone graft substitutes: Failures and hopes. J Eur Ceram Soc. 2012; 32:2663–2671.

5. Ishikawa K. Bone substitute fabrication based on dissolution-precipitation reactions. Materials (Basel). 2010; 3:1138–1155.

6. LeGelos RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991; 15:1–201.
7. Kanayama K, Sriarj W, Shimokawa H, Ohya K, Doi Y, Shibutani T. Osteoclast and osteoblast activities on carbonate apatite plates in cell cultures. J Biomater Appl. 2011; 26:435–449.

8. Ellies LG, Nelson DG, Featherstone JD. Crystallographic structure and surface morphology of sintered carbonated apatites. J Biomed Mater Res. 1988; 22:541–553.

9. Doi Y, Shibutani T, Moriwaki Y, Kajimoto T, Iwayama Y. Sintered carbonate apatites as bioresorbable bone substitutes. J Biomed Mater Res. 1998; 39:603–610.

10. Ishikawa K, Matsuya S, Lin X, Lei Z, Yuasa T, Miyamoto Y. Fabrication of low crystalline B-type carbonate apatite block from low crystalline calcite block. J Ceram Soc Jpn. 2010; 118:341–344.

11. Nagai H, Kobayashi-Fujioka M, Fujisawa K, Ohe G, Takamaru N, Hara K, et al. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J Mater Sci Mater Med. 2015; 26:99.

12. Fujisawa K, Akita K, Fukuda N, Kamada K, Kudoh T, Ohe G, et al. Compositional and histological comparison of carbonate apatite fabricated by dissolution-precipitation reaction and Bio-Oss®
. J Mater Sci Mater Med. 2018; 29:121.
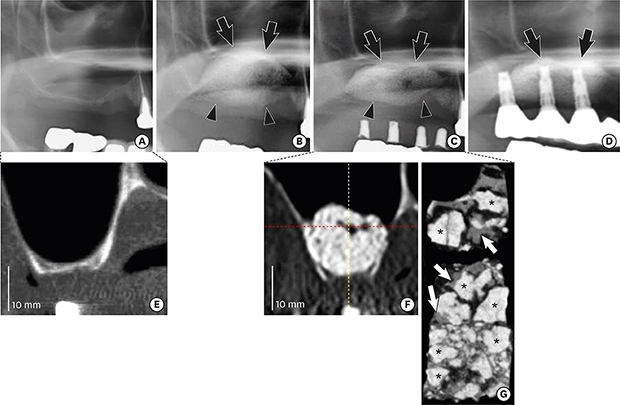
13. Kudoh K, Fukuda N, Kasugai S, Tachikawa N, Koyano K, Matsushita Y, et al. Maxillary sinus floor augmentation using low crystalline carbonate apatite granules with simultaneous implant installation: first-in-human clinical trial. J Oral Maxillofac Surg. 2019; 77:985.e1–985.11.
14. Lin X, Matsuya S, Nakagawa M, Terada Y, Ishikawa K. Effect of molding pressure on fabrication of low-crystalline calcite block. J Mater Sci Mater Med. 2008; 19:479–484.

15. Troedhan A, Schlichting I, Kurrek A, Wainwright M. Primary implant stability in augmented sinuslift-sites after completed bone regeneration: a randomized controlled clinical study comparing four subantrally inserted biomaterials. Sci Rep. 2014; 4:5877.

16. Alsaadi G, Quirynen M, Michiels K, Jacobs R, van Steenberghe D. A biomechanical assessment of the relation between the oral implant stability at insertion and subjective bone quality assessment. J Clin Periodontol. 2007; 34:359–366.

17. Johansson B, Bäck T, Hirsch JM. Cutting torque measurements in conjunction with implant placement in grafted and nongrafted maxillas as an objective evaluation of bone density: a possible method for identifying early implant failures? Clin Implant Dent Relat Res. 2004; 6:9–15.

18. Dos Anjos TL, de Molon RS, Paim PR, Marcantonio E, Marcantonio E Jr, Faeda RS. Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: a prospective, randomized and controlled split-mouth clinical trial. Int J Oral Maxillofac Surg. 2016; 45:1556–1563.

19. O'Sullivan D, Sennerby L, Meredith N. Measurements comparing the initial stability of five designs of dental implants: a human cadaver study. Clin Implant Dent Relat Res. 2000; 2:85–92.
20. Tabassum A, Meijer GJ, Walboomers XF, Jansen JA. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin Oral Implants Res. 2014; 25:487–492.

21. Deppe H, Mücke T, Wagenpfeil S, Hölzle F. Sinus augmentation with intra- vs extraorally harvested bone grafts for the provision of dental implants: clinical long-term results. Quintessence Int. 2012; 43:469–481.
22. Kim YK, Yun PY, Kim SG, Kim BS, Ong JL. Evaluation of sinus bone resorption and marginal bone loss after sinus bone grafting and implant placement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e21–8.

23. Hieu PD, Chung JH, Yim SB, Hong KS. A radiographical study on the changes in height of grafting materials after sinus lift: a comparison between two types of xenogenic materials. J Periodontal Implant Sci. 2010; 40:25–32.

24. Zerbo IR, Zijderveld SA, de Boer A, Bronckers AL, de Lange G, ten Bruggenkate CM, et al. Histomorphometry of human sinus floor augmentation using a porous beta-tricalcium phosphate: a prospective study. Clin Oral Implants Res. 2004; 15:724–732.

25. John HD, Wenz B. Histomorphometric analysis of natural bone mineral for maxillary sinus augmentation. Int J Oral Maxillofac Implants. 2004; 19:199–207.
26. Sartori S, Silvestri M, Forni F, Icaro Cornaglia A, Tesei P, Cattaneo V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res. 2003; 14:369–372.

27. Tadjoedin ES, de Lange GL, Bronckers AL, Lyaruu DM, Burger EH. Deproteinized cancellous bovine bone (Bio-Oss) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. J Clin Periodontol. 2003; 30:261–270.

28. Hallman M, Sennerby L, Lundgren S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int J Oral Maxillofac Implants. 2002; 17:635–643.
29. Galindo-Moreno P, Moreno-Riestra I, Avila G, Fernández-Barbero JE, Mesa F, Aguilar M, et al. Histomorphometric comparison of maxillary pristine bone and composite bone graft biopsies obtained after sinus augmentation. Clin Oral Implants Res. 2010; 21:122–128.

30. Moy PK, Lundgren S, Holmes RE. Maxillary sinus augmentation: histomorphometric analysis of graft materials for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 1993; 51:857–862.

31. Kim Y, Nowzari H, Rich SK. Risk of prion disease transmission through bovine-derived bone substitutes: a systematic review. Clin Implant Dent Relat Res. 2013; 15:645–653.













 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download