1. Van Coevorden AM, Coenraads PJ, Svensson A, Bavinck JN, Diepgen TL, Naldi L, et al. European Dermato-Epidemiology Network (Eden). Overview of studies of treatments for hand eczema-the EDEN hand eczema survey. Br J Dermatol. 2004; 151:446–451. PMID:
15327553.

2. Thyssen JP, Johansen JD, Linneberg A, Menné T. The epidemiology of hand eczema in the general population--prevalence and main findings. Contact Dermatitis. 2010; 62:75–87. PMID:
20136890.
3. Diepgen TL, Agner T, Aberer W, Berth-Jones J, Cambazard F, Elsner P, et al. Management of chronic hand eczema. Contact Dermatitis. 2007; 57:203–210. PMID:
17868211.

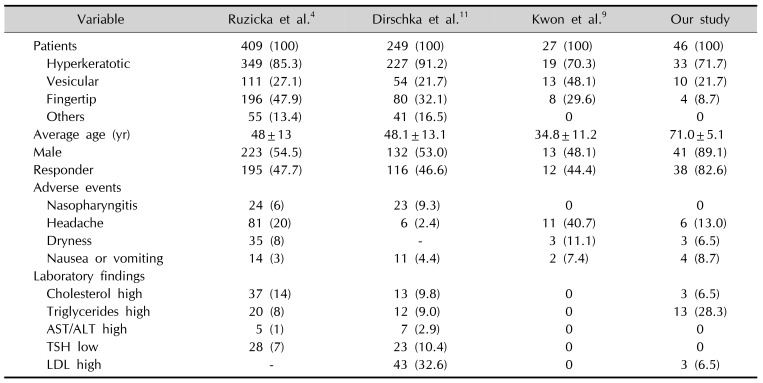
4. Ruzicka T, Lynde CW, Jemec GB, Diepgen T, Berth-Jones J, Coenraads PJ, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol. 2008; 158:808–817. PMID:
18294310.

5. King T, McKenna J, Alexandroff AB. Alitretinoin for the treatment of severe chronic hand eczema. Patient Prefer Adherence. 2014; 8:1629–1634. PMID:
25525339.
6. Ruzicka T, Larsen FG, Galewicz D, Horváth A, Coenraads PJ, Thestrup-Pedersen K, et al. Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: results of a randomized, double-blind, placebo-controlled, multicenter trial. Arch Dermatol. 2004; 140:1453–1459. PMID:
15611422.

7. Prakash AV, Davis MD. Contact dermatitis in older adults: a review of the literature. Am J Clin Dermatol. 2010; 11:373–381. PMID:
20812765.
8. Politiek K, Christoffers WA, Coenraads PJ, Schuttelaar MA. Alitretinoin and acitretin in severe chronic hand eczema; results from a retrospective daily practice study. Dermatol Ther. 2016; 29:364–371. PMID:
27146260.

9. Kwon HI, Kim JE, Ko JY, Ro YS. Efficacy and safety of alitretinoin for chronic hand eczema in Korean patients. Ann Dermatol. 2016; 28:364–370. PMID:
27274636.

10. Fowler JF, Graff O, Hamedani AG. A phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of alitretinoin (BAL4079) in the treatment of severe chronic hand eczema refractory to potent topical corticosteroid therapy. J Drugs Dermatol. 2014; 13:1198–1204. PMID:
25607554.
11. Dirschka T, Reich K, Bissonnette R, Maares J, Brown T, Diepgen TL. An open-label study assessing the safety and efficacy of alitretinoin in patients with severe chronic hand eczema unresponsive to topical corticosteroids. Clin Exp Dermatol. 2011; 36:149–154. PMID:
21070335.

12. Bissonnette R, Worm M, Gerlach B, Guenther L, Cambazard F, Ruzicka T, et al. Successful retreatment with alitretinoin in patients with relapsed chronic hand eczema. Br J Dermatol. 2010; 162:420–426. PMID:
19906075.

14. Diepgen TL, Elsner P, Schliemann S, Fartasch M, Köllner A, Skudlik C, et al. Deutsche Dermatologische Gesellschaft. Guideline on the management of hand eczema ICD-10 Code: L20. L23. L24. L25. L30. J Dtsch Dermatol Ges. 2009; 7 Suppl 3:S1–S16.

15. Al-Dhubaibi MS, Settin AA. The effectiveness of alitretinoin for the treatment of chronic hand eczema: a meta-analysis. Int J Health Sci (Qassim). 2018; 12:70–79.
16. Schmitt-Hoffmann AH, Roos B, Schoetzau A, Leese PT, Meyer I, van de Wetering J, et al. Oral alitretinoin: a review of the clinical pharmacokinetics and pharmacodynamics. Expert Rev Clin Pharmacol. 2012; 5:373–388. PMID:
22943117.

17. Krejci-Manwaring J, McCarty MA, Camacho F, Carroll CL, Johnson K, Manuel J, et al. Adherence with topical treatment is poor compared with adherence with oral agents: implications for effective clinical use of topical agents. J Am Acad Dermatol. 2006; 54(5 Suppl):S235–S236. PMID:
16631951.

18. Tanei R. Atopic dermatitis in the elderly. Inflamm Allergy Drug Targets. 2009; 8:398–404. PMID:
20025588.

19. Schmith GD, Singh R, Gomeni R, Graff O, Hamedani AG, Troughton JS, et al. Use of longitudinal dose-response modeling to support the efficacy and tolerability of alitretinoin in severe refractory chronic hand eczema (CHE). CPT Pharmacometrics Syst Pharmacol. 2015; 4:255–262. PMID:
26225249.

20. Pesante-Pinto JL. Clinical pharmacology and the risks of polypharmacy in the geriatric patient. Phys Med Rehabil Clin N Am. 2017; 28:739–746. PMID:
29031340.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download