Abstract
Objective
Carotid artery stenting is helpful in patients with carotid artery stenosis and is a common method of treatment. However, data on the neurological consequences that might arise from, especially Asian patients after CAS is not enough. The purpose of this study was to investigate the outcome and prognostic factors affecting CAS patients.
Methods
From January 2013 to June 2018 it was enrolled 97 patients who underwent CAS with severe carotid artery stenosis in a single institution. We retrospectively reviewed neurologic complications such as restenosis, ipsilateral or contralateral stroke, and hyperperfusion during the 6-month follow-up period.
Results
There were no complication occured during the procedure in all 97 patients. Neurologic complications occurred in 30 patients (30.9%) after the procedure, and ipsilateral stroke 6 (6.2%), contralateral stroke 9 (9.4%), restenosis 2 (2.1%) and hyperperfusion 13 respectively. One of them had died (1.0%), of which the rest were discharged after symptoms improve. On univariate analysis, DM and pre-op NIHSS score was associated with the risk of CAS complication, exclusively. On the binary logistic regression for risk factors, DM (OR 0.144, 95% CI [0.029–0.718]), history of radiotheraphy (OR 36.103, 95% CI [1.009–1291.789]) and preoperative NIHSS (OR 1.266, 95% CI [1.059–1.513]) showed independent risk factors associated with post procedural neurological complications, statistically.
Carotid artery stenosis accounts for 20 percent of all ischemic strokes in Western countries.11) Carotid artery stsenting (CAS) is emerging as an alternative to classic carotid endarterectomy (CEA) to treat carotid artery stenosis.1)11) Additionally, improvements in endovascular technique and the development of protective devices have dramatically reduced CAS complications. Postoperative complications such as intracranial hemorrhage (ICH), cerebral hyperperfusion syndrome (CHS), stroke, and restenosis after CEA have been well documented, but few reports of neurological outcomes have been made in patients with CAS especially in Asian population. Therefore, the purpose of this study is to study the neurological results of patients receiving CAS from a single institution.1)23)
From January 2013 to June 2018, 97 patients who received CAS for carotid stenosis registered for the study from a single institution. Neurologists and vascular surgeons selected patients suitable for CAS. We performed digital subtraction angiography (DSA) to estimate the degree of stenosis. According to ECST criteria, the indications for CAS were more than 70% (n = 65 (63.16%) with symptom (transient ischemic attack (TIA) or stroke at the site supplied by the stenotic artery) or in asymptomatic cases, more than 70% (n = 32 (36.84%)) on DSA. Our institution's routine douplex ultrasound tests conducted follow-up management regularly six months after stent insertion. After CAS, neurological complications such as ipsilateral or contralateral stroke, resenosis, and hyperperfusion were recorded, analyzed. And the characteristics and conditions before and after procedure was also recorded and reviewed as well.
In Nov. 1999, our CAS program was started at the single neurovascular center. For internal quality control, we have created a computer-based database that includes all patients with carotid stenosis with CAS or carotid vascularization. Patient data was utilized until June 2018 for current analysis. All patients provided consent based on information on procedures and anonymous data collection and processing. All patients with or without symptoms were treated from the time of registration. The anatomical qualification criteria have been tested in all patients by CT angiography or MRA in our institution. If not already given with any anticoagulants, all patients were treated with 300 mg of clopidogrel and 500 mg of acetylsalicylic acid at least 24 hours prior to the procedure. After the procedure, 100 mg of acetylsalicylic acid and 75 mg of clopidogrel were given continuously for more than four weeks. Patients were then advised to take 100 milligrams of acetyl salicylate for life.
In our institution, only local anesthesia was used when performing CAS. Bolus of heparin (5000 IU) was administered in a standard manner. Whenever anatomically possible, we used an embolic protection device for periprocedural neuroprotection. All procedures were performed by a single neurointerventionist with at least 5 years of experience. Also, seven days before the procedure, patients received aspirin and Plavix for optimal treatment in addition to CAS. And within 24 hours prior to and after procedure, all patients were received neurological assessment by neurologist. An independent neurologist evaluated with the National Institutes of Health Stroke Scale and the modified Rankin Scale. First, entryways for angiography were secured through the right femoral artery and 5-F sheath was used. If you secure the fore-aft-side of the CCA through the catheter selection, and if stenosis is verified, we replaced it with guided-wire long enough that can reach the statistical CCA and ECA. Special care must be taken to avoid inappropriate contact between the stenosis and wire when the tip of the guided wired reaches near the CCA. If the Micro-wire and Microcatheter are successfully located in the stenosis, the delivery device must be over the microwire and past the stenosis. The length of the stent should be sufficient to extend from the CCA to the ICA in most cases to fully cover the stenosis. The diameter of the stent must match the CCA in order to properly match the wall adaptation of all carotid segments. The stent should be positioned slightly further than the desired stenosis position and should be pulled out before the position to be placed is reached, in order to reduce the possibility of a forward swivel movement. Atropine should be prepared for bradycardia at all times. Angiography should be performed on both cervical ICAs and intracranial circulation to evaluate stenosis, vasospasm, dissection, and cerebral blood flow. In this case, it is important to compare with the angiogram performed before the operation because the distal emboli are difficult to detect. After performing CAS through this procedure, it is necessary to pay attention to the complications that may arise from enlarging the diameter of the lesion by dilating the stent.
Data are expressed according to the properties of the variable. Continuous variables are presented as mean and standard deviation. Categorical variables are presented as frequency and percentage. In order to compare two groups, we performed the two-simple t-test or chi-square test (Fisher`s exact test) as appropriate. Logistic regression analysis was used to identify the factors to predict neurologic complications after CAS and the result were expressed as odds ratio (OR) with 95% confidence interval (CI). A p-value less than 0.05 was considered statistically significant and all statistical analyses were conducted using SPSS (version 24; IBM, Armonk, NY, USA).
We retrospectively analyzed 97 patients who underwent CAS with carotid artery stenosis from 2013 to 2018. The total number of patients enrolled in the study was 97 (15 women (15.5%) and 82 men (84.5%)) and the average age was 71.3 year old. Procedure was successfully performed in all 97 patients and protection devices were used in all cases. 65 patients were diagnosed with TIA or cerebral infarction before CAS, and 32 patients who received CAS were diagnosed with stenosis from regular health check-ups without any symptoms. The occurrence of selected patient characteristics is shown in Table 1, which lists the general risk factors of the study population. We discovered that the most frequently found medical history is hypertension in 72 (74.2%) patients, history of stroke in 55 (56.7 %) patients, and hyperlipidemia in 36 (37.1%) patients. Furthermore, the medical history showed that 27 (27.8%) patients had history of coronary artery disease, 13 (13.4%) had history of transient ischemic attack, 32 (33.0%) patients had diabetes mellitus (DM), 33 (34.0%) patients were smokers, 18 (18.6%) had a history of cancer and 3 (3.1%) patients and a history of radiotherapy. In this study, only hyperlipidemia was a risk factor associated with carotid artery stenosis, statistically (p=0.009). Other characteristics were not related to the occurrence of the symptoms (Table 1).
Neurologic complications were observed in 30 patients (30.9%) after the procedure, and ipsilateral stroke 6 (6.2%), contralateral stroke 9 (9.4%), restenosis 2 (2.1%) and hyperperfusion 13 (13.4%) occurred (Table 2). One of them died from ICH caused by severe hyperperfusion (Figure 1), while the rest were discharged after symptoms improved. Two patients (2.1%) with restenosis were identified by Duplex Ultrasonography during follow up period.
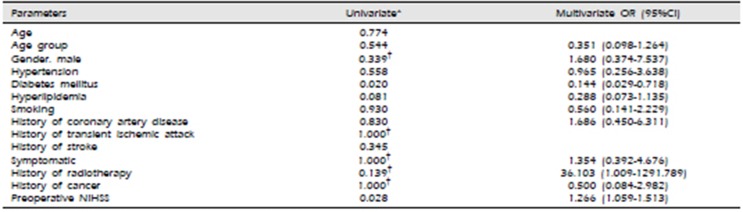
On univariate analysis, gender, age, hypertension, hyperlipidemia, and presence of coronary artery disease, history of radiotherapy, or smoking did not show a significant association with procedural neurological events (Table 3). DM and pre-op NIHSS score was associated with the risk of CAS complication, exclusively. On the binary logistic regression for risk factors, DM (OR 0.144, 95% CI [0.029–0.718]), history of radiotheraphy (OR 36.103, 95% CI [1.009–1291.789]) and preoperative NIHSS (OR 1.266, 95% CI [1.059–1.513]) showed independent risk factors associated with post procedural neurological complications, statistically. Interestingly, DM showed association with the risk of CAS complications in negative OR (Table 3).
According to American Guidelines for the Prevention of Stroke, carotid recanalization, CEA, or carotid artery stenting, is recommended for patients who experience ischemic stroke or transient cerebral ischemic stroke within 6 months (symptomatic patient) underlying ipsilateral carotid stenosis as demonstrated by catheter-based angiography or noninvasive imaging.20) CAS has recently been widely used as a reliable treatment that can replace CEA, and there are significantly more CAS-based treatments in our institution compared to CEA in patients with carotid artery stenosis. In particular, for asymptomatic carotid artery stenosis in stroke patients, recanalization is recommended to reduce the surgical risk to 3% or less. In spite of these great advantages, it sometimes provides a disastrous cerebral deficit such as contrast-induced encephalopathy, ischemic stroke, and CHS.20) CAS was used to expand the diameter of a stenotic lesion using a stent, and pre- and post-operative data on neurological events including stroke, seizure, ICH, CHS, or changes in neurological status were collected.
Many authors have described CHS as a primary complication of carotid recanalization procedure.17) CHS is defined in several ways such as: 1) Severe headache, seizures, mental deterioration, and / or development of local neurological symptoms; 2) no additional ischemic lesion in imaging diagnosis of the brain; 3) Increased cerebral blood flow in the ipsilateral hemisphere, increased blood flow in the contralateral hemisphere, or increased cerebral artery flow rate value of > 100% compared with preoperative velocity, all postoperatively.7)14) According to Moulakakis et al, the incidence of CHS and ICH after CAS was 1.16% (range, 0.44% to 11.7%) and 0.74% (range, 0.36% to 4.5%), respectively and CHS may be fatal once an intracranial hemorrhage occurs.17) In this study, 13 cases (13.4%) were shown in all cases conforming to the above definition of HPS, but HPS with fetal ICH reported was 1 case (1.1%) in our institution. This increase in morbidity is associated with increased blood flow through the cerebral artery after the procedure and inadequate arterial blood pressure control.2) Although we found this phenomenon to be present after CEA for the first time, many authors have reported that post carotid angioplasty and stenting have similar results.7)21)
The most common time period for occurrence of CHS is several hours to several days after the procedure.20) Ogasawara suggested that the onset time of CHS among patients who received CAS reached its peak within 12 hours after the procedure. Mental deterioration, confusion, and headache are the most common symptoms of CHS, among which headache usually occurs on the ipsilateral side of the arterial artery, often degree of moderate to severe, and characterized by pulsatile and migraine symptoms.4) Cerebral edema is a neurological deficit of secondary CHS, which is usually transient. These are mostly derived from the cerebral cortex, including hemiplegia, hemiparesis, hemianopsia, aphasia, and obtundation, and also epileptic disorders such as generalized or focal seizures. Other symptoms such as movement disorders, visual impairment, cognitive impairment, and psychotic disorders are rare, but have reported in CHS patients as well.19) And some of the untreated patients may die.17)
The pathogenesis and risk factors of CHS are not fully known, but uncontrolled hypertension seems to be the most important cause of CHS.5) It stimulates the occipital-parietal region more noticeably because of the sympathetic nerves that innervate very little on the vertebrobasilar circulation.22) This suggests that impaired cerebral autoregulation, baroreceptor-reflex breakdown, and an axon-like trigeminovascular reflex contribute to the pathogenesis of CHS.16)17) Another serious concern is the intake of antiplatelet and statins, which is recommended for patients with carotid stenosis before and after procedure.5) These drugs can increase the risk of cerebral hemorrhage and CHS, especially after carotid stenting.20)
ICH is the most disasteric event secondary to hyperperfusion, which is also associated with CAS.17) ICH can cause vomiting or change of mentality because of increased pressure in the cranium.1)
It is important to note that ICH after CAS is difficult to prevent because it appears to occur within few hours without symptoms and mostly unavoidable and even fatal.17) There are several risk factors, including preoperative hypertension, bilateral carotid disease or contralateral carotid occlusion as well as impaired cerebrovascular reserve, which were associated with the formation of ICH after the procedure.7) It is known that ICH, which appears immediately after CAS, is caused by the rupture of the perforating artery in the basal ganglia hence the sudden exposure of normal perfusion pressure pathophysiologically.17) Rate of morbidity and mortality are high in both patients who treated with open surgery or endovascular repair, therefore it is crucial to prevent such a catastrophic event after the procedure.3)7)
Stroke occurs more commonly after CAS than after CEA. The periprocedural strokes in CREST were most commonly minor, ipsilateral to the treated artery, and ischemic in type and occurred twice as frequently in the CAS arm.9) Major stroke occurred in 0.6% (13/2272), indicative of the very low overall complication rate observed in the trial.8)9) According to Michael et al, complications that occurred during the carotid stent placement or within a 30-day period following placement were recorded and the definitions were defined as follows: (1) TIA, any neurological deficit (either ocular or cerebral) that resolved completely within 24 hours; (2) minor stroke, any new neurological deficit (either ocular or cerebral) that persisted for 24 hours and that either resolved completely within 7 days or increased the National Institutes of Health stroke scale 3 points; (3) major stroke, any new neurological deficit that persisted after 30 days and increased the National Institutes of Health stroke scale by 3 points.12)
There is evidence of a higher risk of stroke and mortality after CAS in patients with symptomatic patients than in asymptomatic patients, but it has not yet been studied how certain symptoms affect previously symptomatic patients after CAS procedure.12)
In this study, we analyzed all patients who underwent CAS to identify potential clinical risk factors for neurologic complications after CAS procedure. The significance of this study is that no previous studies analyzing the effects of CAS on post-procedural results have been reported, particularly in Asia, and based on evidence that the risk of stroke and death after CEA depends not only on symptoms but also on the type of presenting event.11)12) In some studies, age was identified as an independent risk factor for neurologic complications after CAS procedure, but DM and preoperative NIHSS were found to be related to our study. Hyperlipidemia has also been described as a risk factor in patients with complication after CAS in a number of studies. Comparable with recent studies in Qureshi et al., with that in univariate analysis, we found no association between the hyperlipidemia and postprocedural neurological deficit. Diabetes (DM) is an independent risk factor for cardiovascular disease and stroke and has a high incidence of diabetes among people undergoing carotid revascularization.12)13)15) However, it is well known that the risk of morbidity and mortality or the risk of late stroke is not greater after CAS in diabetic patients.12) Renato et al. reported that the presence of diabetes was associated with an increased periprocedural risk, but did not show any additional risk as a result of long-term follow-up and had a higher rate of restenosis in diabetic patients.18) Therefore, these studies are not comparable to our results. Studies suggesting that post-surgical risk for DM patients may be higher after CEA.6) And this may be helpful in selecting the appropriate technique for carotid revascularization in patients best suited to the procedure type. In some studies, clinical symptoms have also been known to be potential risk factors for periprocedural complications following CAS, but not in accord with our study. However, this association is reported to be consistently insignificant in other studies as well, therefore we could come to a conclusion that it is not significant.10) The cause of this conflicting result may be a small number of patients.
Several limitations should be pointed out. There may be some neurological complications that the even patient has not noticed would have been missed. The size of the data was small. Without a randomized control group, no clear comparison of CEA or other medical treatment can be made. Since the current data is collected as a single center experience, the results cannot be generalized in a simple way.
CAS is a relatively safe and reliable procedure that provides long-term outcome for patients with carotid artery stenosis. However, it is necessary to recognize the incidence of complications after CAS procedure in carotid artery stenosis patients, and careful observation of possible complications after procedure is necessary for patients with a history of pretreatment radiotherapy or high NIHSS score.
References
1. Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004; 5. 43(9):1596–1601. PMID: 15120817.
2. Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, Franklin IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 2011; 2. 41(2):229–237. PMID: 21131217.

3. Cheung RT, Eliasziw M, Meldrum HE, Fox AJ, Barnett HJ. North American Symptomatic Carotid Endarterectomy Trial G. Risk, types, and severity of intracranial hemorrhage in patients with symptomatic carotid artery stenosis. Stroke. 2003; 8. 34(8):1847–1851. PMID: 12829862.
4. Coutts SB, Hill MD, Hu WY. Hyperperfusion syndrome: toward a stricter definition. Neurosurgery. 2003; 11. 53(5):1053–1058. discussion 8–60. PMID: 14580271.
5. De Rango P. Cerebral hyperperfusion syndrome: the dark side of carotid endarterectomy. Eur J Vasc Endovasc Surg. 2012; 4. 43(4):377. PMID: 22289610.

6. Parlani G, De Rango P, Cieri E, Verzini F, Giordano G, Simonte G, Isernia G, et al. Diabetes is not a predictor of outcome for carotid revascularization with stenting as it may be for carotid endarterectomy. J Vasc Surg. 2012; 1. 55(1):79–89. PMID: 22056251.

7. Galyfos G, Sianou A, Filis K. Cerebral hyperperfusion syndrome and intracranial hemorrhage after carotid endarterectomy or carotid stenting: A meta-analysis. J Neurol Sci. 2017; 10. 15. 381:74–82. PMID: 28991720.

8. Hill M, Brooks W, Mackey A, Clark WM, Meschia JF, Morrish WF, et al. Stroke After Carotid Stenting and Endarterectomy in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Circulation. 2012; 7. 01. 17(7):587–596.

9. Hill MD, Brooks W, Mackey A, Clark WM, Meschia JF, Morrish WF, et al. Stroke after carotid stenting and endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Circulation. 2012; 12. 18. 126(25):3054–3061. PMID: 23159552.

10. Hofmann R, Niessner A, Kypta A, Steinwender C, Kammler J, Kerschner K, et al. Risk score for peri-interventional complications of carotid artery stenting. Stroke. 2006; 10. 37(10):2557–2561. PMID: 16990579.

11. Hung CS, Lin MS, Chen YH, Huang CC, Li HY, Kao HL. Prognostic Factors for Neurologic Outcome in Patients with Carotid Artery Stenting. Acta Cardiol Sin. 2016; 3. 32(2):205–214. PMID: 27122951.
12. Kastrup A, Groschel K, Schulz JB, Nagele T, Ernemann U. Clinical predictors of transient ischemic attack, stroke, or death within 30 days of carotid angioplasty and stenting. Stroke. 2005; 4. 36(4):787–791. PMID: 15705938.

13. Knur R. Cerebral Hyperperfusion Syndrome following Protected Carotid Artery Stenting. Case Rep Vasc Med. 2013; 2013:207602. PMID: 23956924.

14. Lieb M, Shah U, Hines GL. Cerebral hyperperfusion syndrome after carotid intervention: a review. Cardiol Rev. 2012; Mar-Apr. 20(2):84–89. PMID: 22183061.
15. Mathur A, Roubin GS, Iyer SS, Piamsonboon C, Liu MW, Gomez CR, et al. Predictors of stroke complicating carotid artery stenting. Circulation. 1998; 4. 97(13):1239–1245. PMID: 9570193.

16. McCabe DJ, Brown MM, Clifton A. Fatal cerebral reperfusion hemorrhage after carotid stenting. Stroke. 1999; 11. 30(11):2483–2486. PMID: 10548688.

17. Moulakakis KG, Mylonas SN, Sfyroeras GS, Andrikopoulos V. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg. 2009; 4. 49(4):1060–1068. PMID: 19249185.

18. Adegbala O, Martin KD, Otuada D, Akinyemiju T. Diabetes Mellitus with Chronic Complications in Relation to Carotid Endarterectomy and Carotid Artery Stenting Outcomes. J Stroke Cerebrovasc Dis. 2017; 1. 26(1):217–224. PMID: 27810149.

19. Ogasawara K, Yamadate K, Kobayashi M, Endo H, Fukuda T, Yoshida K, et al. Postoperative cerebral hyperperfusion associated with impaired cognitive function in patients undergoing carotid endarterectomy. J Neurosurg. 2005; 1. 102(1):38–44.

20. Siroos B, Harirchian MH, Kazemi Khaledi A, Ghaffarpour M, Golshani S. Cerebral Hyperperfusion Syndrome, an Unusual but Disastrous Complication of Carotid Recanalization: A Case Report. J Stroke Cerebrovasc Dis. 2018; 2. 27(2):e17–e19. PMID: 28988884.

21. Tan GS, Phatouros CC. Cerebral hyperperfusion syndrome post-carotid artery stenting. J Med Imaging Radiat Oncol. 2009; 2. 53(1):81–86. PMID: 19453532.

22. van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005; 12. 4(12):877–888. PMID: 16297845.

23. Wholey MH, Wholey M, Mathias K, Roubin GS, Diethrich EB, Henry M, et al. Global experience in cervical carotid artery stent placement. Catheter Cardiovasc Interv. 2000; 6. 50(2):160–167. PMID: 10842380.

Fig. 1
A 79-year-old male was admitted for left side weakness due to right border zone infarction (A). After performing digital subtraction angiography, he was diagnosed with a 78.4% stenosis of the right internal carotid artery (B). He underwent right CAS procedure without periprocedural complication (C). Three hours following CAS, a computerized tomography of the brain reveals a large right-hemisphere hematoma, with midline shift and intraventricular hemorrhage (D).
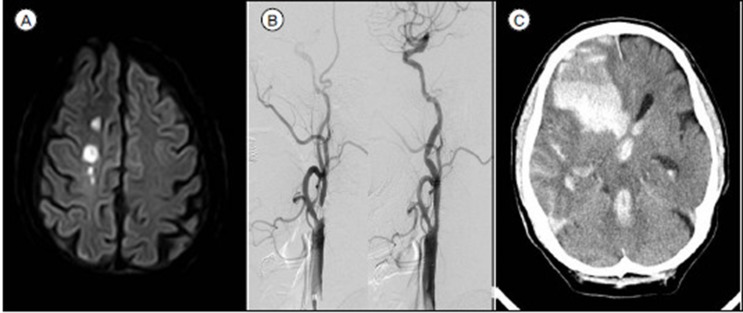
Table 1
Patients characteristics with and without symptoms
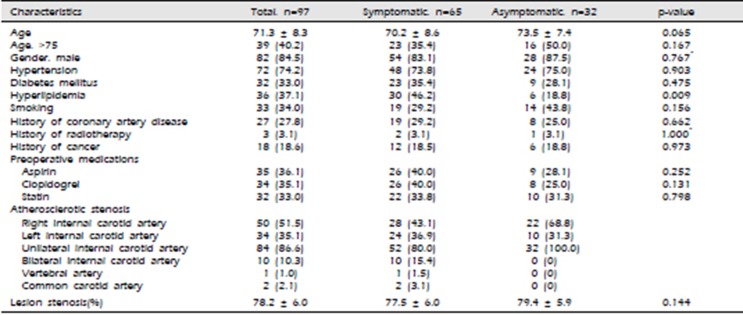
Table 2
Neurologic complications after carotid artery stenting

| Neurologic complications | N=30 |
|---|---|
| Ipsilateral ischemic stroke | 6 (6.2) |
| Contralateral ischemic stroke | 9 (9.3) |
| Restenosis | 2 (2.1) |
| Hyperperfusion | 13 (13.4) |
Table 3
Univariate and multivariate analysis of risk factors and neurologic complications




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download