MATERIALS AND METHODS
We retrospectively analyzed the angiographic and clinical records of all patients who underwent EVT for RTAs at our institution from April 2006 to December 2017. Only patients with aneurysms sized ≤3 mm were included in the study. The sizes of their aneurysms and parent arteries were measured automatically and manually on two-dimensional digital subtraction angiography working views. Dissecting or bleb aneurysms, aneurysms associated with a brain arteriovenous malformation, and traumatic or mycotic aneurysms were excluded. All procedures were performed by three experienced neurointerventionist (J.K.K., J.I.L., and T.H.L.). In most cases, the patients were eligible for both treatment modalities. Factors, including patient characteristics, aneurysm features, and operator's preferences, were taken into account in the decision-making process. The patients with posterior circulation aneurysms were primarily offered endovascular therapy. Conversely, the patients with a large intraparenchymal hematoma underwent open surgery for aneurysm clipping and simultaneous clot evacuation and decompression. Elderly patients with multiple comorbidities or poor neurological grades were often preferentially offered an endovascular procedure. Aneurysms with an unfavorable neck-to-dome ratio were either clipped or coiled using stent or balloon assistance. Owing to the retrospective nature of the present study, the need for obtaining informed consent was waived by our Institutional Review Board.
Patient population and aneurysm morphologies
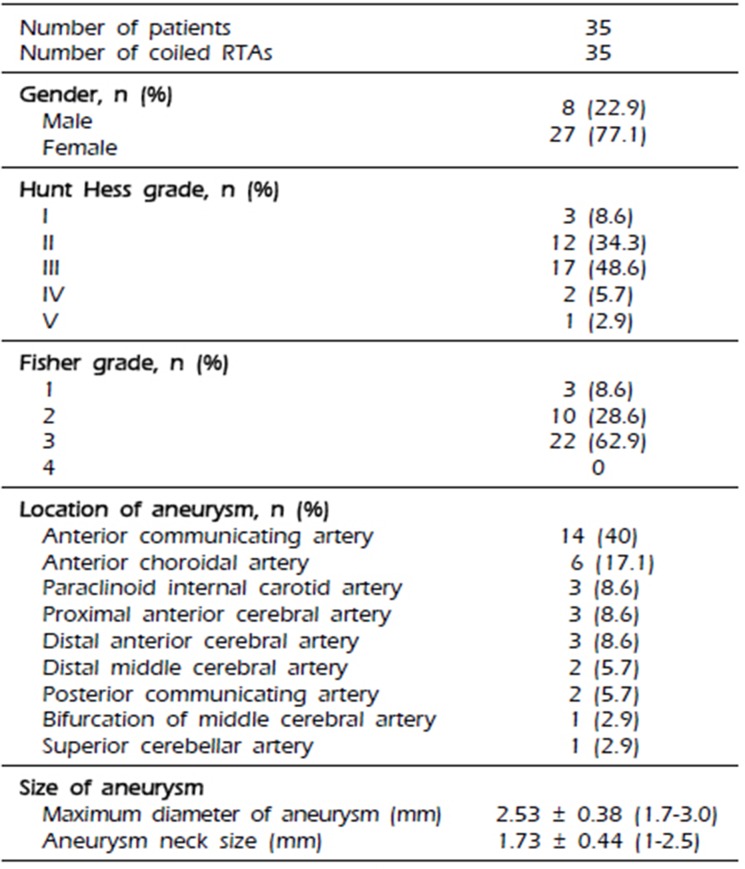
The demographic data of the patients and characteristics of the aneurysms are provided in
Table 1. Thirty-five RTAs in 35 patients were treated endovascularly, and there were 8 men and 27 women with a mean age of 56 years (SD, 14.4; range, 29–81). The aneurysm and neck sizes ranged from 1.7 to 3.0 mm (mean, 2.53 mm) and from 1 to 2.6 mm (mean, 1.73 mm), respectively. The average aspect ratio of RTAs was 1.51 (SD: 0.34). Of the 35 RTAs, 14 were located at the anterior communicating artery; 6 at the anterior choroidal artery; 3 each at the paraclinoid internal carotid artery, proximal anterior cerebral artery, and distal anterior cerebral artery; 2 each at the distal middle cerebral artery and posterior communicating artery; and 1 each at the bifurcation of the middle cerebral artery and superior cerebellar artery. Four patients had at least one additional aneurysm. All patients were clinically assessed at admission using the Hunt and Hess grades: 3 patients (8.6%), grade I; 12 patients, grade II (34.3%); 17 patients, grade III (48.6%); 2 patients, grade IV (5.7%); and 1 patient, grade V (2.9%). Further, the Fisher grade in all patients was assessed: 3 patients, grade 1 (8.6%); 10 patients, grade 2 (28.6%); and 22 patients, grade 3 (62.9%).
Table 1
Patient demographic data and characteristics of the aneurysms
|
Number of patients |
35 |
|
Number of coiled RTAs |
35 |
|
Gender, n (%)
|
|
|
Male |
8 (22.9) |
|
Female |
27 (77.1) |
|
Hunt Hess grade, n (%)
|
|
|
I |
3 (8.6) |
|
II |
12 (34.3) |
|
III |
17 (48.6) |
|
IV |
2 (5.7) |
|
V |
1 (2.9) |
|
Fisher grade, n (%)
|
|
|
1 |
3 (8.6) |
|
2 |
10 (28.6) |
|
3 |
22 (62.9) |
|
4 |
0 |
|
Location of aneurysm, n (%)
|
|
|
Anterior communicating artery |
14 (40) |
|
Anterior choroidal artery |
6 (17.1) |
|
Paraclinoid internal carotid artery |
3 (8.6) |
|
Proximal anterior cerebral artery |
3 (8.6) |
|
Distal anterior cerebral artery |
3 (8.6) |
|
Distal middle cerebral artery |
2 (5.7) |
|
Posterior communicating artery |
2 (5.7) |
|
Bifurcation of middle cerebral artery |
1 (2.9) |
|
Superior cerebellar artery |
1 (2.9) |
|
Size of aneurysm
|
|
|
Maximum diameter of aneurysm (mm) |
2.53 ± 0.38 (1.7-3.0) |
|
Aneurysm neck size (mm) |
1.73 ± 0.44 (1-2.5) |

Endovascular procedure
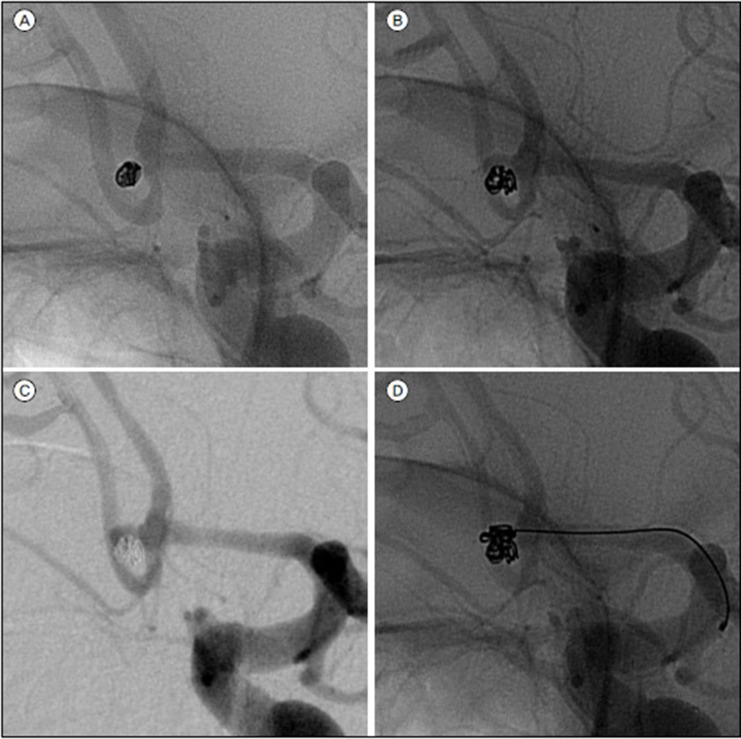
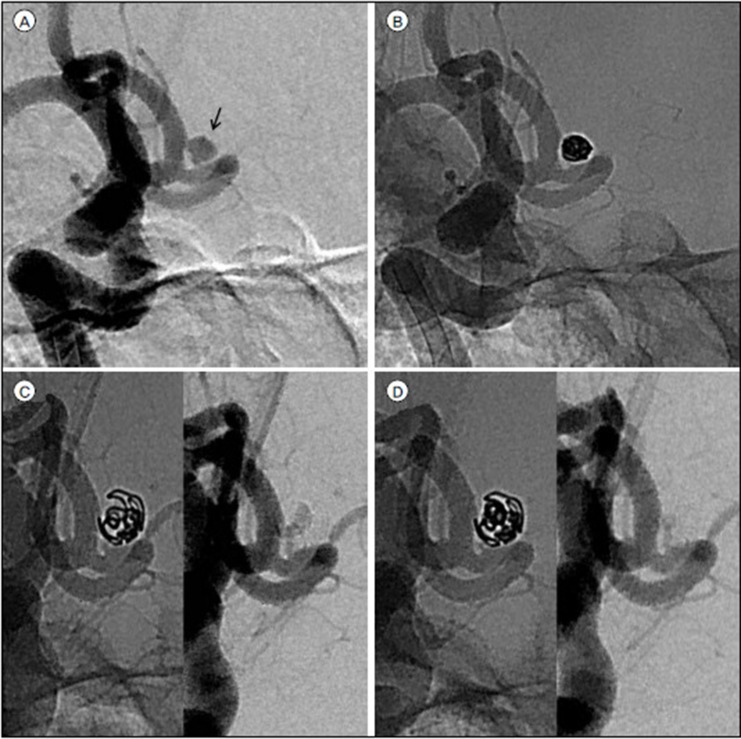
In general, EVT was performed as soon as possible after subarachnoid hemorrhage, regardless of clinical condition. No antiplatelet premedication was prescribed in any patients because of the risk of rebleeding. Coiling of the aneurysms was performed under local anesthesia, and intravenous bolus injection of standard heparin was not performed during the procedure. However, drip infusion of heparin was conducted employing catheter pressure infusion systems with continuous infusion of 1000 U of heparin per 1000 mL of saline. The aim of the coiling procedure was to obtain a packing of the aneurysm as attenuated as possible. Various technical measures and precautions were taken to overcome specific problems. Remodeling techniques using a balloon or stent or multiple catheters were unavailable for the aneurysms treated during the early part of our series. However, we have recently favored these remodeling techniques to achieve higher postoperative occlusion rates. In the case of intraprocedural thromboembolic complication, various strategies for thrombolysis were applied, such as mechanical thrombolysis and intra-arterial or intravenous aggrastat or heparin infusion during or after the procedure.
Immediately after the procedure, a complete neurological examination was performed on all patients by a vascular neurosurgeon. Furthermore, all patients underwent a non-enhanced brain computed tomography (CT) scan to evaluate possible hemorrhagic complications. Unless the thromboembolic complication occurred or a stent was used, further antiplatelet or anticoagulant medications were not administered. The indications for long-term antiplatelet therapy of acetylsalicylic acid and/or clopidogrel were as follows: formation of a thrombus at the end of the coil or thromboembolic events, stent-assisted coiling, and coil protrusion into the parent artery.
Clinical and angiographic follow-ups
The immediate and follow-up angiographic results were analyzed. Occlusion was classified as follows: “complete” when no contrast filling of the aneurysmal sac and neck was observed, “near-complete” when contrast filling of the aneurysmal neck was slow, or “partial” when any degree of contrast filling was observed within the aneurysmal sac. The clinical results were assessed upon discharge from the hospital or at the last visit using the modified Rankin scale (mRS) score as follows: 0, no symptoms at all; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death. A poor clinical outcome was defined as an mRS score of 3 to 6. Complications were defined as all adverse events related to the procedure, which were studied retrospectively using medical and operative reports. Periprocedural rebleeding (PPR) included both IPR and ERB. IPR was defined as clinically and angiographically evident rupture during the procedure. ERB was defined as expanding hemorrhage with worsening of the patients' condition within 30 days after coiling; diagnosis was confirmed using CT when it showed an increased amount of hemorrhage compared with immediate postprocedural CT.
Go to :

DISCUSSION
EVT of RTAs has been reported to be technically difficult primarily because of the presence of frequently broad-based aneurysms with a shallow dome and complex shape, difficulty in maintaining microcatheter stability, difficulty in achieving complete coil packing, and higher risk of IPR. The introduction of new microcatheters and microguidewires with improved trackability, pushability, torque, and novel hydrophilic coatings has facilitated navigation into RTAs. Furthermore, the availabilities of various soft coils may have contributed to the higher success rate of small aneurysm coiling without IPR.
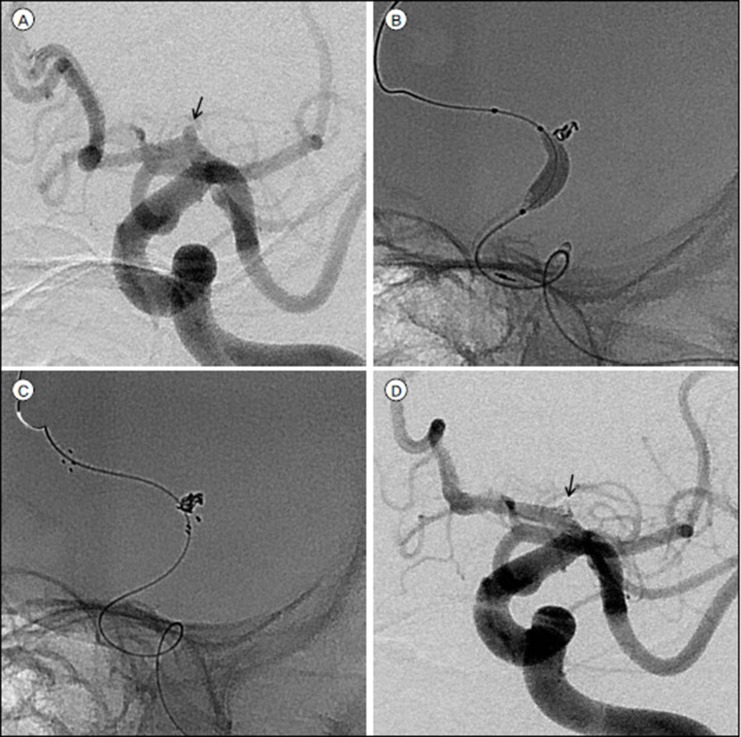
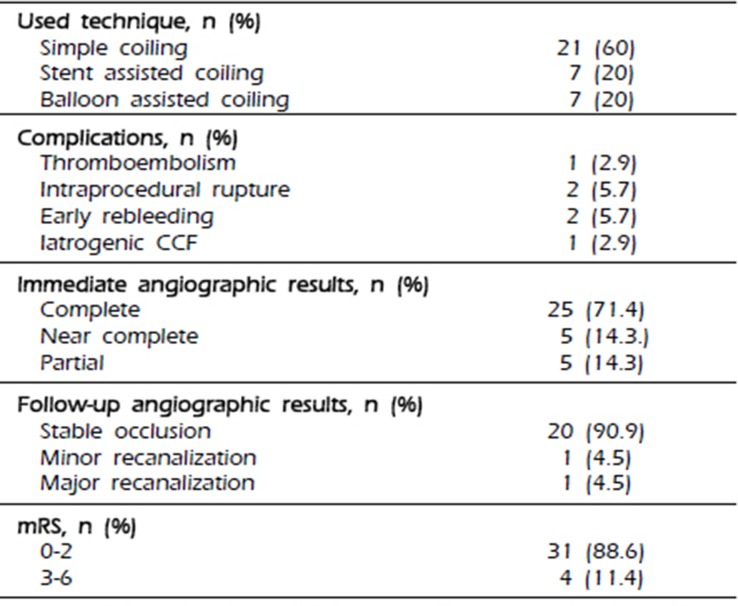
In this series of 35 endovascularly treated RTAs, immediate post-procedural angiograms showed complete occlusion in 71.4% of the patients, near-complete occlusion in 14.3%, and partial occlusion in 14.3%. The procedure-related complications included two cases of IPR (5.7%), one case of thromboembolic event (2.9%), and two cases of ERB, which needed recoiling (5.7%). At the end of the follow-up, 88.6% of the patients showed favorable outcomes (mRS score, 0–2). Follow-up conventional angiography (mean, 468 days) showed stable occlusion in 90.9%, minor recanalization in 4.5%, and major recanalization, which required recoiling, in 4.5%. In our series, an anterior choroidal aneurysm has a much higher ratio than a posterior communicating aneurysm (17.1% vs 5.7%). Because an anterior choroidal aneurysm tends to rupture easily even in relatively small sizes, and there is our policy of surgical treatment for a fetal-type posterior communicating aneurysm. In addition, we have recently favored the remodeling techniques using a balloon or stent or multiple catheters to achieve higher postoperative occlusion rates, and to prevent the kick-back movement of the microcatheter, especially in RTA cases.
Only a few studies have compared between the outcomes of coiling of RTAs and ruptured larger aneurysms. We attempted to determine the risk and effectiveness of coiling of RTAs by comparing them with those of coiling of larger ones reported in previous studies. In the International Subarachnoid Aneurysm Trial (ISAT), which is the largest prospective randomized study on endovascular and neurosurgical treatments of ruptured intracranial aneurysms of all sizes, follow-up angiography (mean, 12 months) showed complete occlusion in 66% of aneurysms, neck remnant in 26%, and partial occlusion in 8%.13) In that series, favorable clinical outcomes were achieved in 73.9% of the patients. Further, the rate of IPR was 1.9%. Except IPR that occurred more frequently in our series, the results of our study are similar to those of the ISAT. Van Rooji et al. compared between the clinical outcomes after coiling of 196 very small aneurysms (size, ≤3 mm) in 187 patients and those of 1099 larger aneurysms in 1006 patients.
19) They found that 76% (149/196) of the very small aneurysms were ruptured, and 94.9% were completely occluded; further, 87.2% of the patients showed favorable outcomes at follow-up. IPR occurred in 15 of the 196 (7.7%) very small aneurysms and in 36 of the 1099 (3.6%) larger aneurysms. They concluded that coiling of aneurysms sized ≤3 mm was technically feasible with a lower complication rate comparable with that of larger aneurysms. However, the rate of IPR was much higher in the very small aneurysms than in the larger aneurysms. As detailed above, several studies on coiling of very small aneurysms reported a high rate of IPR but concluded that coiling of such aneurysms was technically feasible with a low complication rate and good clinical outcomes.
12)19) In other reports, IPR did not actually increase the overall morbidity and mortality rates.
4)19) Our research findings support their hypothesis. In our series, the two patients who experienced IPR showed favorable neurological outcomes (mRS score, 0-2) and could function independently.
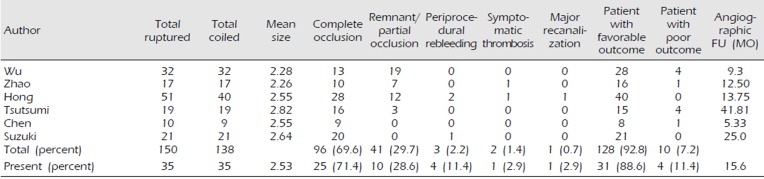
We summarized the reported studies in which more than 10 patients with RTAs were treated with EVT in
Table 3.
5)8)17)18)21)23) These studies included 150 aneurysms, of which 138 were coiled. Similar to our results, 96 of the 138 (69.6%) coiled aneurysms were completely occluded, while 41 (29.7%) were partially occluded or had a small remnant. There were three cases of IPR and one case of symptomatic thrombosis during the procedure. We compared the results of our study and those of the previous studies we reviewed in
Table 3. The other results are similar to ours, except for PPR. Our series showed a rate of PPR of 11.4%, while the reviewed studies revealed only a total rate of 2.2%. Most of the previous studies reviewed did not report ERB. However, we think that the importance of ERB in coiling of RTAs cannot be overemphasized. Prior studies on ERB after coiling have reported rupture rates of 1.4% to 2.6%.
9)10)16) Incomplete embolization, adjacent hematoma, small aneurysms, and post-procedural anticoagulation have been reported to be associated with ERB after coiling. The Cerebral Aneurysm Rerupture After Treatment study reported that the risk of post-treatment rebleeding was closely associated with the degree of aneurysm occlusion.
10) However, we experienced two cases of ERB (5.7%), despite both aneurysms being completely embolized during the procedure. The presence of adjacent hematoma is a strong, independent risk factor of ERB, and a thrombosed pseudoaneurysm and hematoma could cause early reopening and rebleeding.
16) In our series, both cases of ERB had no adjacent hematoma. Conversely, Cho et al., who reported on early recurrent hemorrhage after coil embolization in ruptured intracranial aneurysms, hypothesized another possibility that rebleeding with intracranial hemorrhage (ICH) may be caused by delayed hemorrhage or propagation of the initial ICH owing to vulnerability of the parenchyma adjacent to the ICH, rather than by rebleeding of the coiled aneurysm.
6) ERB after coiling of a ruptured aneurysm and intraprocedural rupture are major concerns because the associated mortality rates are high. When an RTA is accompanied by adjacent hematoma, decisions regarding EVT should be taken only after carefully considering the possibility of PPR. Therefore, it may be better to consider surgical clipping of an RTA with adjacent hematoma as the first option to prevent PPR. Yamaki et al. conducted a meta-analysis on the outcomes of EVT of very small aneurysms (size, ≤3 mm).
22) They reported a complete occlusion rate of 88% after coil embolization of RTAs. Among their patients diagnosed with RTAs treated with EVT, 74% showed favorable neurological outcomes. The rates of PPR, symptomatic thromboembolism, recanalization, and retreatment were 9%, 4%, 9%, and 7%, respectively. They reported a high rate of PPR similar to ours. In retrospect, two cases of ERB and one case of IPR occurred in the early period of this series at our institution. It is assumed that the lack of experience of aneurysm coiling and undeveloped coil technology at that time might have partially contributed to the higher rate of PPR. It is believed that small aneurysms are more prone to IPR because there is less room for manipulation of endovascular devices.
15) Because stiffness is inversely proportional to the length of the coil segment, the stiffness of the initial coil segment would be higher in smaller aneurysms than in larger aneurysms; therefore, the risk of rupture would be higher in the former than in the latter.
2)
Table 3
Literature review of endovascular treatment of RTAs
|
Author |
Total ruptured |
Total coiled |
Mean size |
Complete occlusion |
Remnant/partial occlusion |
Periprocedural rebleeding |
Symptomatic thrombosis |
Major recanalization |
Patient with favorable outcome |
Patient with poor outcome |
Angiographic FU (MO) |
|
Wu |
32 |
32 |
2.28 |
13 |
19 |
0 |
0 |
0 |
28 |
4 |
9.3 |
|
Zhao |
17 |
17 |
2.26 |
10 |
7 |
0 |
1 |
0 |
16 |
1 |
12.50 |
|
Hong |
51 |
40 |
2.55 |
28 |
12 |
2 |
1 |
1 |
40 |
0 |
13.75 |
|
Tsutsumi |
19 |
19 |
2.82 |
16 |
3 |
0 |
0 |
0 |
15 |
4 |
41.81 |
|
Chen |
10 |
9 |
2.55 |
9 |
0 |
0 |
0 |
0 |
8 |
1 |
5.33 |
|
Suzuki |
21 |
21 |
2.64 |
20 |
0 |
1 |
0 |
0 |
21 |
0 |
25.0 |
|
Total (percent) |
150 |
138 |
|
96 (69.6) |
41 (29.7) |
3 (2.2) |
2 (1.4) |
1 (0.7) |
128 (92.8) |
10 (7.2) |
|
|
Present (percent) |
35 |
35 |
2.53 |
25 (71.4) |
10 (28.6) |
4 (11.4) |
1 (2.9) |
1 (2.9) |
31 (88.6) |
4 (11.4) |
15.6 |

This study is limited by its retrospective design, patient selection bias, small cohort, and the fact that it was conducted at a single institution. Further, direct comparison with microsurgical clip reconstruction of ruptured tiny saccular aneurysms is difficult given the very small number of published series. However, our findings suggest that EVT of RTAs appears to be a valid treatment strategy given suitable levels of institutional and operator expertise. Moreover, appropriate patient selection for endovascular versus surgical intervention could lead to better outcomes in patients with RTAs.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download