Abstract
Purpose
This study sought to describe the different clinical features and presentations of primary ocular toxoplasmosis in a setting not demonstrating an outbreak of disease.
Methods
This was a retrospective review of patients presenting to uveitis management services in Auckland and Hamilton, New Zealand between 2003 to 2018 with uveitis and positive toxoplasmosis immunoglobulin M serology.
Results
We identified 16 patients with primary acquired toxoplasmosis infection and ocular involvement. The mean age was 53 years. Systemic symptoms were reported in 56% (9 / 16). Visual acuity was reduced to 20 / 30 or less in 50% of patients (8 / 16). A single focus of retinitis without a pigmented scar was the salient clinical feature in 69% (11 / 16). Optic nerve inflammation was the sole clinical finding in 19% (3 / 16). Bilateral arterial vasculitis was the sole clinical finding in 13% (2 / 16). A delay in the diagnosis of toxoplasmosis of more than two weeks occurred in 38% (6 / 16) due to an initial alternative diagnosis. Antibiotic therapy was prescribed in all cases. Vision was maintained or improved in 69% (11 / 16) at the most recent follow-up visit (15 months to 10 years). Relapse occurred in 69% (11 / 16), typically within four years from the initial presentation.
Conclusions
Primary ocular toxoplasmosis presenting in adulthood is a relatively uncommon cause of posterior uveitis in New Zealand. This condition should be considered in any patient presenting with retinitis or optic nerve inflammation without a retinochoroidal scar. This disease tends to relapse; thus, close follow-up is required.
Toxoplasmosis is a well-recognized cause of posterior uveitis. Initially, it was thought that most primary infections occurred in utero; however, more recent evidence suggests that almost two-thirds of infections are acquired postnatally [1]. In the absence of the classical retinochoroidal scar, the diagnosis of primary ocular toxoplasmosis in adulthood can be difficult to make, as it can mimic other causes of uveitis. The aim of our study was to describe the different clinical features and presentations of primary ocular toxoplasmosis in a setting not experiencing an outbreak of disease.
We conducted a retrospective observational review of all patients who presented to uveitis management services in Auckland and Hamilton, New Zealand between 2003 and 2018 with primary ocular toxoplasmosis. This study adhered to the tenets of the Declaration of Helsinki, and the New Zealand Ethics Committee advised us that ethical approval was not required for this study.
Patients were included in this research if they 1) presented with an inaugural episode of uveitis that was compatible with primary ocular toxoplasmosis (i.e., absence of a retinochoroidal scar), 2) showed positive toxoplasma immunoglobulin M (IgM) serology, and 3) other causes of uveitis were excluded.
Vision was reported using Snellen acuity. The quantification of toxoplasma immunoglobulin G (IgG) and IgM levels was performed using enzyme immunoassay techniques. The techniques differed between the two regions and across hospital and community laboratory services. These included the VIDAS (bioMérieux, Marcy l'Etoile, France) and AxSYM and ARCHTIECT (Abbott Laboratories, Chicago, IL, USA) automated systems. IgG sensitivities and specificities and IgM specificities, respectively, for all three techniques exceeded 93%, while IgM sensitivities varied between 62% and 90% depending on the system used [23]. Toxoplasma IgM was reported as positive or negative by the laboratory services in Auckland and Hamilton and numerical values were not provided. Although the quantification of toxoplasma IgG levels was reported, the values and units used to express antibody levels differed depending on the testing method used. For the purpose of this study, IgG levels were reported as elevated or normal.
Patient demographics and clinical findings are presented in Table 1. A history to support a recent exposure to toxoplasmosis, mostly from cats, was documented in 44% (7 / 16). Thirteen percent (2 / 16) were immunocompromised, which included one patient on methotrexate for rheumatoid arthritis and another on chemotherapy for acute myeloid leukaemia.
The most common finding (11 / 16, 69%) was a single focus of retinitis, which was located in the peripheral retina in two-thirds of patients (Fig. 1). This included one patient who initially presented with hypertensive anterior uveitis who later developed a focus of retinitis at three weeks following his initial presentation, with toxoplasma identified from an aqueous sample. Of those presenting with retinitis, raised intraocular pressure (>21 mmHg) was observed in 28% (3 / 11), with an associated vasculitis observed in 36% (4 / 11).
Optic nerve inflammation was the sole clinical finding in 19% (3 / 16) manifesting as papillitis alone or with juxtapapillary retinochoroiditis (Fig. 2). This result included findings in two patients who initially presented with papillitis and vitritis, with normal brain magnetic resonance imaging scans and laboratory tests (including chest X-ray) negative for tuberculosis, sarcoidosis, and bartonella. In two of the patients who presented with papillitis alone, a juxtapapillary infiltrate developed a fortnight following the initial presentation, with serology and vitreous fluid positive for toxoplasma.
Two patients presented with bilateral vasculitis without retinochoroiditis, with Kyrieleis plaques observed in one patient (Fig. 3). These two patients were included amongst the 38% (6 / 16) who experienced a delay in the diagnosis of toxoplasmosis by more than two weeks. The remaining four included two patients who presented with optic nerve inf lammation (as described above), one who presented with anterior uveitis and subsequently developed retinochoroiditis, and a single case wherein retinitis was initially assumed to be herpetic in origin.
An intraocular sample was obtained in 44% (7 / 16), with toxoplasma polymerase chain reaction (PCR) findings positive in six out of the cases, including in two patients who had toxoplasma DNA detected from an aqueous specimen. Vitreous fluid was obtained from five patients, of whom four showed positive PCR results. This included one patient who failed to improve after 6 weeks of no treatment and one who relapsed prior to elective vitrectomy for symptomatic vitreous debris.
Antibiotic therapy was prescribed in all cases and those who were immunocompetent were also prescribed a tapering course of oral steroids. A delay in the initiation of antibiotic treatment of more than 2 weeks occurred in 50% (8 / 16), which included two patients who failed to improve after six weeks without treatment, while the remainder's lack of response was attributed to a delay with diagnosis.
Vision was maintained or had improved in 69% (11 / 16) at follow-up (15 months to 10 years). Identified reasons for poor vision most commonly included reversible causes such as persistent vitreous debris and cataract. Retinochoroiditis affecting the macula resulted in a single line of visual loss for one patient. Patients with juxtapapillary retinochoroiditis had residual visual deficits; however, visual acuity was preserved. Relapse occurred in 69% (11 / 16), typically within the first four years of follow-up. Two patients remain on lifelong antibiotic prophylaxis for recurrent relapses.
Primary ocular toxoplasmosis presenting in adulthood has been observed to occur in populations following an outbreak of Toxoplasma gondii infection [45]. This study, representing the largest reported case series from New Zealand, highlights the importance of recognizing primary toxoplasmosis as a cause of posterior uveitis in a nonendemic setting as well. The diagnosis of ocular toxoplasmosis infection (reactivation) is usually made with relative ease based on the clinical findings of an inflammatory focus adjacent to a pigmented scar with vitreous inflammation and vasculitis. Optic nerve involvement is not uncommon [6]. In patients with primary ocular toxoplasmosis, there are no pathognomonic features and thus the diagnosis can be difficult to make. This can translate to a delay in diagnosis, which occurred in 38% of our patients.
Although there were no defining features to help diagnose primary ocular toxoplasmosis, we did note some interesting observations. First, the mean age of our cohort was 53 years, with comparable ages reported in other studies of acquired toxoplasma ocular disease [45]. The higher mean age is in contrast with the ages of those who present with early-onset or ‘congenital’ reactivation, as these individuals are typically young adults between 20 and 30 years of age. With respect to the clinical findings, 88% had unilateral disease with a focus of retinochoroiditis commonly observed in the peripheries. In contrast, patients with early-onset infection can have recurrences in both eyes, with a focus of inflammation typically seen at the posterior pole [6].
Differentiating retinitis associated with primary toxoplasmosis from other infective causes of uveitis such as herpetic causes can be difficult. Research has shown that primary ocular toxoplasmosis is the most common other diagnosis that mimics acute retinal necrosis (ARN). A study by Balansard et al. [7] showed that 62% of patients labelled as having ARN were subsequently diagnosed instead with toxoplasmosis. Specific clinical findings can sometimes help differentiate the two processes, with toxoplasmosis presenting as a necrotising retinitis with a district border, smooth contoured edges, and sans retinal haemorrhages. Vitritis is usually moderate even in those who are immunosuppressed, and progression is usually more indolent as compared with that seen during the rapid destructive process seen in ARN. However, the clinical findings can be difficult to appreciate when there is significant intraocular inflammation, and, in such cases, laboratory confirmation is required, especially in those who are immunocompromised.
Serological testing is useful in the work-up of posterior uveitis, with a negative result excluding toxoplasmosis as a potential cause. However, a negative result is of limited use in patients who are immunosuppressed, and, in such cases, an intraocular specimen should be obtained. We acknowledge that there are limitations to using serology to diagnose active toxoplasma infection and this represents a weakness of our study. Detectable IgM levels are indicative of a recent infection and typically remain elevated for one year, with a variable rate of decline [6]. Toxoplasma IgG levels are typically elevated within the first few weeks following infection and can remain so for several years. Toxplasma IgG seropositivity is prevalent in most countries including New Zealand. Of 500 serum samples taken from pregnant women in Auckland, 33% were IgG-positive and 2.4% were IgM-positive, while an IgG seropositivity rate of 43% was reported in Hamilton [89]. In our case study, all patients were toxoplasma IgM-positive and 88% had raised IgG levels on presentation. Although rising serum IgG antibodies can be used to support recent infection, serial testing of IgG was either not done routinely or the follow-up test was performed at a different laboratory site (possibly with different testing techniques used), where the units used to express antibody levels also may have differed. However, the observation of a satisfactory response to anti-toxoplasmosis therapy in those treated supported the diagnoses of toxoplasma infection. Of note, one patient who initially responded to combined antibiotic and steroid therapy had a significant increase in the size of retinitis after he discontinued oral antibiotics and continued steroid treatment. Also, two patients with suspected immune-related papillitis developed a focus of retinitis on unopposed immunosuppression. In both cases, the area of retinitis had settled with the addition of antiparasitic therapy.
We observed several atypical presentations of toxoplasmosis including two patients presenting with vasculitis without retinochoroiditis. One of these patients underwent a vitreous biopsy, which was negative. However, the clinical sign of Kyrieleis arteritis was highly suggestive of toxoplasmosis. Although detectable toxoplasma IgM may simply represent a coincidental finding in both cases, it is possible that the inflammation observed in these atypical cases was not directly attributable to intraocular infection but rather represented an immunoreactive phenomenon, as postinfectious uveitis has been reported following streptococcal infection and viral illness [1011]. The other possibility is that PCR testing of the vitreous produced a false-negative result and that the bilateral uveitis did indeed represent primary intraocular toxoplasmosis infection.
Intraocular sampling enables both PCR testing and calculation of the Goldmann-Witmer coefficient, although the latter technique is not available for use in our institutions. In our centre, vitreous or aqueous sampling was not automatically performed in all patients but instead was dependent on the preference of the treating clinician. An intraocular specimen was obtained in seven patients, with toxoplasma DNA detected in six. The PCR method, which involves the amplification of the toxoplasmosis B1 gene, is considered less sensitive than for viral causes of uveitis with sensitivities ranging from 27% to 85% [1213141516]. Harper et al. [17] reported a sensitivity of 67%, which is lower than the 82% reported for the detection of herpetic viruses. The authors could not offer comments on the relative sensitivity in different ocular fluids. Furthermore, the sensitivity has been shown to be affected by the degree of inflammation. Fardeau et al. [13] found that larger areas of retinitis were more likely to yield a positive result. Labalette et al. [15] noted aqueous PCR was positive in 60% when the retinitis was greater than three-disc diameters, yet only 25% when the lesions were smaller.
In conclusion, primary ocular toxoplasmosis presenting in adulthood is a relatively uncommon cause of posterior uveitis in New Zealand. The clinical findings can vary and a high index of suspicion should be raised in any patient presenting with retinitis or optic nerve inflammation without a retinochoriodal scar. The condition does tend to relapse and thus close follow-up is required.
Figures and Tables
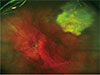 | Fig. 1This wide-field image focused at the posterior pole shows a single focus of retinitis (resolving) with areas of chorioretinal atrophy in the left superior temporal retina. |
Notes
References
1. Gilbert RE, Stanford MR. Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol. 2000; 84:224–226.
2. Calderaro A, Piccolo G, Peruzzi S, et al. Evaluation of Toxoplasma gondii immunoglobulin G (IgG) and IgM assays incorporating the newVidia analyzer system. Clin Vaccine Immunol. 2008; 15:1076–1079.
3. Murat JB, Dard C, Fricker Hidalgo H, et al. Comparison of the Vidas system and two recent fully automated assays for diagnosis and follow-up of toxoplasmosis in pregnant women and newborns. Clin Vaccine Immunol. 2013; 20:1203–1212.
4. Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol. 2010; 128:28–32.
5. Burnett AJ, Shortt SG, Isaac-Renton J, et al. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology. 1998; 105:1032–1037.
6. Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Exp Ophthalmol. 2013; 41:95–108.
7. Balansard B, Bodaghi B, Cassoux N, et al. Necrotising retinopathies simulating acute retinal necrosis syndrome. Br J Ophthalmol. 2005; 89:96–101.
8. Morris A, Croxson M. Serological evidence of Toxoplasma gondii infection among pregnant women in Auckland. N Z Med J. 2004; 117:U770.
9. Zarkovic A, McMurray C, Deva N, et al. Seropositivity rates for Bartonella henselae, Toxocara canis and Toxoplasma gondii in New Zealand blood donors. Clin Exp Ophthalmol. 2007; 35:131–134.
10. de Smet MD. Papillophlebitis and uveitis as a manifestation of post-streptococcal uveitis syndrome. Eye (Lond). 2009; 23:985–987.
11. Lai CC, Chang YS, Li ML, et al. Acute anterior uveitis and optic neuritis as ocular complications of influenza A infection in an 11-year-old boy. J Pediatr Ophthalmol Strabismus. 2011; 48 Online:e30–e33.
12. Fekkar A, Bodaghi B, Touafek F, et al. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2008; 46:1965–1967.
13. Fardeau C, Romand S, Rao NA, et al. Diagnosis of toxoplasmic retinochoroiditis with atypical clinical features. Am J Ophthalmol. 2002; 134:196–203.
14. Montoya JG, Parmley S, Liesenfeld O, et al. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology. 1999; 106:1554–1563.
15. Labalette P, Delhaes L, Margaron F, et al. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol. 2002; 133:506–515.
16. Errera MH, Goldschmidt P, Batellier L, et al. Real-time polymerase chain reaction and intraocular antibody production for the diagnosis of viral versus toxoplasmic infectious posterior uveitis. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1837–1846.
17. Harper TW, Miller D, Schiffman JC, Davis JL. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009; 147:140–147.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download