Abstract
Purpose
To evaluate the efficacy of focal verteporfin photodynamic therapy (PDT) in patients diagnosed with chronic central serous chorioretinopathy (CSC).
Methods
This study enrolled 52 eyes of 52 patients with chronic CSC who had received verteporfin PDT. The laser spot size of 26 eyes covering only the localized hyperfluorescent area in indocyanine green angiography was classified as focal PDT. The PDT spot size of the other 26 eyes covered the total area of retinal pigment epithelial detachment including the leaking point and was defined as conventional PDT. The central subfield thickness and subfoveal choroidal thickness were measured using Heidelberg Spectralis optical coherence tomography before PDT and at months 1, 3, 6, and 12 after PDT.
Results
The mean spot size of the PDT was 1,995 µm in the focal group and 2,995 µm in the conventional group. Central subfield thickness steadily decreased in both groups. The mean baseline subfoveal choroidal thickness for the two groups was 334.95 and 348.35 µm, respectively, with no significant difference (p = 0.602). Subfoveal choroidal thickness decreased significantly to 304.20 µm at 1 month, 284.85 µm at 3 months, 271.60 µm at 6 months, and 265.95 µm at 12 months in the focal group (p < 0.001, p < 0.001, p < 0.001, and p < 0.001, respectively, compared with baseline). In the conventional group, subfoveal choroidal thickness decreased significantly to 318.75, 300, 284, and 272 µm at 1, 3, 6, and 12 months, respectively (p < 0.001, p < 0.001, p < 0.001 and p < 0.001 compared with baseline). There were no significant differences between the two groups in subfoveal choroidal thickness based on PDT spot size at 1, 3, 6, and 12 months (p = 0.633, p = 0.625, p = 0.676, and p =0.755, respectively).
Central serous chorioretinopathy (CSC) is an idiopathic syndrome prevalent in young-to-middle-aged adults. It is characterized by serous retinal detachment of the macula secondary to increased permeability of choroidal vessels and a barrier defect in the retinal pigment epithelium (RPE) [12]. The main pathogenic mechanism involves a breakdown of the outer blood-retinal barrier resulting from defective choroidal circulation [13]. Generally, subfoveal choroid in the eyes of patients with CSC is thicker than in normal eyes because of choroidal vascular hyperpermeability. In acute CSC, patients often complain of blurred vision, micropsia, and metamorphopsia. However, visual acuity is relatively good despite serous retinal detachment. Meanwhile, chronic CSC is often associated with atrophic and degenerative changes of the retina and RPE and, consequently, with decreased visual acuity [456].
Chronic CSC is defined as presence of persistent serous retinal detachment for more than 6 months and widespread areas of leakage following RPE damage. In chronic CSC, persistent macular detachment may trigger degenerative changes in RPE and the neurosensory retina, which result in a poor visual outcome. Therefore, several treatment options have emerged in an attempt to resolve subretinal fluid accumulation and to improve the visual outcomes in patients with chronic CSC.
The advent of fluorescein angiography (FA) and indocyanine green angiography (ICGA) has facilitated diagnosis of CSC. ICGA has been used to evaluate choroidal vasculature and highlight mid-phase multifocal areas of choroidal hyperfluorescence in CSC [1].
Treatment of CSC with ICGA-guided verteporfin photodynamic therapy (PDT) can decrease choroidal vascular hyperpermeability and consequently reduce leakage from the RPE. Therefore, constriction and occlusion of choroidal vessels following treatment decreases choroidal thickness [27].
However, several complications related to full-fluence PDT have been reported, including RPE atrophy, choroidal ischemia, choriocapillaris hypoperfusion, and secondary choroidal neovascularization (CNV) [68]. Modification of PDT parameters, such as irradiation, exposure time, or verteporfin dose, yielded similar anatomical and visual results to conventional PDT, generally with fewer complications [91011]. However, there have been no studies on prognosis of CSC according to PDT spot size.
The purpose of this study was to evaluate the efficacy of focal PDT confined to areas of focal leakage in patients with CSC and to assess the 1-year follow-up results of subfoveal choroidal thickness after PDT.
We conducted a retrospective study of eyes with chronic CSC treated with verteporfin PDT at the Kyung Hee University Medical Center between March 2009 and December 2013. Approval for this retrospective review was obtained from the Institutional Review Board of our institution (2019-05-059). Informed consent was waived due to the retrospective nature of the study.
CSC was defined as detachment of the neurosensory retina from the macula caused by idiopathic or diffuse leakage from the RPE. Leakage from the RPE was detected by FA, and choroidal vascular hyperpermeability was detected by ICGA. Chronic CSC was diagnosed when symptoms of subretinal fluid (SRF) persisted for more than 3 months. Eyes were excluded if another macular abnormality or cause of serous retinal detachment was found, such as neovascular maculopathy (i.e., age-related macular degeneration, polypoidal choroidal vasculopathy, or other secondary CNV), intraocular inflammation, or posterior segment tumor. Eyes were also excluded if another treatment modality, such as laser treatment, had been performed within 3 months before PDT or if there had been previous treatment by intravitreal anti-vascular endothelial growth factor injections or previous PDT.
A total of 52 eyes of 52 patients with chronic CSC who had received verteporfin PDT were included. The patients were divided into two groups according to PDT treatment strategy and spot size: the ‘focal PDT group (group 1)’, covering only the localized hyper-fluorescent area in ICGA and the ‘conventional PDT group (group 2)’, covering the total area of abnormal choroidal vessels including the leakage point (Fig. 1A–1E). Both groups were comprised of 26 patients.
Clinical data and imaging studies were retrieved from the visits that led to PDT and based on routine follow-up visits at approximately 1, 3, 6, and 12 months after PDT, including refraction, best-corrected visual acuity (BCVA, Snellen), slit-lamp biomicroscopy, indirect funduscopy, fundus photography (FF450 plus; Carl Zeiss Meditec, Jena, Germany), optical coherence tomography (OCT; Cirrus HD-OCT 5000, Carl Zeiss Meditec), FA, and ICGA (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Subfoveal choroidal thickness was determined with the enhanced depth imaging OCT technique utilizing the Heidelberg Spectralis OCT device. Choroidal thickness was defined as the distance between the hyper-reflective line corresponding to Bruch's membrane beneath the RPE and the choroidal-scleral interface. Subfoveal choroidal thickness was independently assessed by two masked observers.
Patients were treated using a full dose of verteporfin (6 mg/m2; Visudyne, Novartis Ophthalmics AG, Basel, Switzerland). Verteporfin was infused over 10 minutes, followed by delivery of an activating light dose of 600 mW/cm2 from the laser system (Carl Zeiss, Dublin, CA, USA) over an exposure time of 83 seconds.
Statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA). The changes in central subfield thickness and subfoveal choroidal thickness were evaluated with a repeated measures ANOVA followed by Bonferroni post-hoc tests. Student's t-test was used to compare changes between the two groups. Fisher's exact test was used to compare the incidence of adverse ocular events. For all statistical tests, a p-value less than 0.05 was considered statistically significant.
Between March 2009 and December 2013, 52 patients were included in the study and followed from baseline to 12 months. Both groups included 26 patients. The average age of patients was 52.55 ± 8.96 years in group 1 and 54.65 ± 12.58 years in group 2. The mean BCVA of study eyes at baseline was obtained as logarithm of the minimum angle of resolution (logMAR) 0.34 ± 0.35 and logMAR 0.41 ± 0.37 in groups 1 and 2, respectively. There were no significant differences in demographic or study parameters between the two groups at baseline (Table 1).
The mean spot size for PDT was 1,995 ± 496.81 µm in group 1 and 2,995 ± 991.25 µm in group 2, with significant difference between the groups (p < 0.001).
The mean central subfield thickness was 328.11 ± 102.54 µm at baseline and 204.20 ± 76.17 µm at 12 months after PDT in group 1 (RM-ANOVA, p < 0.001) (Table 2). In group 2, the mean central subfield thickness was 345.88 ± 112.02 µm at baseline and 224.50 ± 83.24 µm at 12 months (RM-ANOVA, p < 0.001) (Table 2). The central subfield thickness steadily decreased in both groups (Fig. 2A–2D). In addition, no recurrent cases were found within 1 year after PDT in either group.
The mean baseline subfoveal choroidal thickness was 334.95 ± 85.03 µm in group 1 and 348.35 ± 109.10 µm in group 2, with no significant difference between the two groups (p = 0.602). The mean subfoveal choroidal thickness decreased significantly to 304.20 µm at 1 month post-PDT, 284.85 µm at 3 months, 271.60 µm at 6 months, and 265.95 µm at 12 months in group 1 (RM-ANOVA, p < 0.001, p < 0.001, p < 0.001, and p < 0.001, respectively compared with baseline). These values were 91%, 85%, 82%, and 79% of baseline at 1, 3, 6, and 12 months, respectively (Table 2). In group 2, these value decreased significantly to 318.75, 300, 284, and 272 µm at 1, 3, 6, and 12 months, respectively (RM-ANOVA, p < 0.001, p < 0.001, p < 0.001 and p < 0.001 compared with baseline). These values were 92%, 86%, 82%, and 78% of baseline at 1, 3, 6, and 12 months, respectively (Table 2). There was no significant difference between the two groups with regard to subfoveal choroidal thickness according to PDT spot size at 1, 3, 6, or 12 months (Student's t-test, p =0.633, p =0.625, p = 0.676, and p = 0.755, respectively).
All eyes in the focal PDT group and the conventional PDT group demonstrated complete absorption of SRF at post-PDT 12 months. The mean time from baseline to complete resolution of SRF was 1.08 ± 0.68 months for all eyes, 1.16 ± 0.71 months in the focal PDT group, and 1.02 ± 0.56 months in the conventional PDT group. No significant difference in resolution time was noted between the two groups (p = 0.421). No recurrent cases were detected within 1 year after PDT in either group.
RPE atrophy in the PDT area was found in both groups at the last follow-up. Observed rates of RPE atrophy were 3.85% (1 / 26 eyes) in the focal PDT group and 11.59% (3 / 26 eyes) in the conventional PDT group, but there was no significant difference between the two groups (Fisher's exact test, p = 0.561). No other systemic side effects, such as cardiovascular events or cerebral vascular accidents, were encountered in the study.
In this study, we investigated subfoveal choroidal thickness and central subfield thickness after PDT in patients diagnosed with chronic CSC and hyperautofluorescent subretinal deposits. The objective was to assess the effect of focal PDT compared with that of conventional PDT.
PDT with verteporfin ameliorated CSC and classic subfoveal CNV secondary to age-related macular degeneration or pathological myopia. In a previous study, the choroidal thickness of CSC patients following PDT was reduced to its normal level, but it did not decrease to its normal level in spontaneously resolved CSC patients [12]. This finding indicated that the healing mechanism of PDT might differ from that of natural approaches. In the PDT group, retinal reattachment may occur subsequent to attenuation of choroidal thickness and concomitant with normalization of subfoveal choroidal thickness. The disparity may explain the relatively lower recurrence rate of CSC after PDT, which led to short-term occlusion of choriocapillaris and long-term choroidal vascular remodeling [1314].
PDT uses laser spot sizes adjusted to cover the lesion area and enhance absorption of subretinal fluid in CSC during infrared diode laser exposure. Thus, it is important to determine the appropriate PDT spot size. If the treatment spot is smaller than expected, CSC may be left untreated, resulting in treatment failure. By contrast, oversized treatment may result in a larger area of damage to the retina, RPE, and choroid than necessary, which may lead to unnecessary loss of visual function [1516]. Several complications associated with PDT have been reported, such as choroidal ischaemia, choriocapillaris hypoperfusion, persistent RPE atrophy, and secondary CNV [17]. There is not PDT protocol for CSC to decrease choroidal vascular hyperpermeability without adverse effects on the choroidal vessels and RPE. There are no standard guidelines determining the size and location of PDT as well as the dose of verteporfin and radiance for CSC patients. Therefore, the decision regarding spot size is critical to maximize the effectiveness and minimize the damage associated with PDT.
Several previous studies found a reduction in subfoveal choroidal thickness after PDT. Particularly, Chan et al. [18] showed persistent changes in choroidal vessels and thickness at 1 year, possibly indicating that half-dose PDT with verteporfin was effective for CSC at least 1 year after treatment. Maruko et al. [19] administered ICG-guided PDT to the entire macula, visualized by enhanced depth imaging OCT, and reported that reduction in choroidal thickness was evident both at 1 week and 1 month after PDT. Pryds and Larsen [20] analyzed the effect of PDT on choroidal thickness in small areas of extrafoveal focal pigment epithelial leakage locally and in the fovea, at considerable distance from the treated area. The choroid was markedly thinner after treatment not only in the area of the fundus where PDT was applied, but also under the fovea. Furthermore, the relative decrease in choroidal thickness was more pronounced under the fovea than in the area exposed to PDT. Thus, it is important to determine the area treated with PDT to ensure lasting therapeutic effects.
To the best of our knowledge, this retrospective study is the first to exclusively address the association between PDT spot size and subfoveal choroidal thickness after PDT in eyes of patients with chronic CSC. Many retrospective studies and case series have reported that PDT treatment for CSC decreased choroidal vascular hypermeability, reduced leakage from the RPE level, and enhanced the therapeutic outcome. Maruko et al. [21] reported that half-dose PDT with verteporfin reduced choroidal vascular hyperpermeability and choroidal thickness concurrently based on choroidal evaluation using ICGA and enhanced depth imaging OCT. This finding suggested that choroidal thickness was a standard parameter for successful treatment outcome.
Comparing the therapeutic effect of PDT in the two groups, all patients harbored hyperautofluorescent subretinal deposits before treatment, and retinal detachment was resolved in all patients after a single session of PDT. No recurrent cases were found within 1 year after PDT in either group. Moreover, subfoveal choroidal thickness also decreased significantly during the first year in both groups. Analysis of choroidal thickness after PDT revealed a meaningful decrease at 1, 3, 6, and 12 months compared with initial thickness in both groups. Thus, focal verteporfin PDT for CSC significantly decreased the subretinal fluid and sufoveal choroidal thickness, and the results were comparable to those of conventional PDT. Thus, there were no significant differences between the two groups with regard to subfoveal choroidal thickness based on PDT spot size. Notably, the two groups showed similar patterns of decrease in choroidal thickness after PDT therapy. This study demonstrated that focal PDT was comparable to conventional PDT in clinical efficacy. RPE atrophy in the PDT area was found in both groups at the last follow-up. RPE atrophy was reported in 3.85% (1 / 26 eyes) of the focal PDT treatment group and 11.59% (2 / 26 eyes) of the conventional PDT treatment group. Although there were no significant differences in adverse events, considering a previous report that electrophysiological and laboratory studies have demonstrated transient reduction in macular function after conventional PDT, potential retinal damage caused by PDT may be minimized by reducing unnecessary laser exposure. Although there was no significant difference in RPE atrophy or secondary CNV due to the limitations of a 12-month follow-up study, long-term follow-up analysis of this study may clarify the merits of focal PDT. Therefore, our study provides a guideline for optimum PDT spot size, preventing needless treatment and minimizing treatment-related side effects.
This study had several limitations, including a small sample size and a short-term follow up. Further study is needed to evaluate a significantly larger number of patients over a long-term follow-up period lasting more than 3 years to ascertain the long-term effects of focal PDT treatment. The manual measurement of choroidal thickness was another drawback of our study. However, previous studies reported that measurements of choroidal thickness using enhanced depth imaging-OCT showed good reproducibility and repeatability [22]. Further, errors in measurement may not be critical based on similar normal values reported in our study and in previous studies.
In conclusion, our results demonstrated that focal verteporfin PDT for CSC, confined to areas of localized hyperfluorescent leakage in ICGA, resulted in significant decrease in subretinal fluid and sufoveal choroidal thickness as well as conventional PDT during the 1-year follow-up. Even simple measurements of subfoveal choroidal thickness on OCT images provide objective insights into management of CSC. Although prospective studies with larger numbers of patients and long-term follow-up are needed to accurately determine treatment effects and establish the protocol for focal PDT therapy in patients with chronic CSC, this study elucidates the criteria for optimal PDT spot size to maximize the effectiveness and minimize the damage of treatment.
Figures and Tables
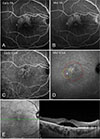 | Fig. 1Example of a chronic central serous chorioretinopathy patient who was treated with conventional photodynamic therapy (PDT). (A,B) Fluorescein angiogram before PDT showed focal leakages from the retinal pigment epithelium at the macula. Indocyanine green angiogram before PDT showed hyperfluorescence secondary to vascular hyperpermeability at the macula. (C,D) Yellow circle indicates ‘focal PDT’ area and red circle indicates ‘conventional PDT’ area in middle phase indocyanine green angiogram. (E) Spectral-domain optical coherence tomography images before PDT. FA = fluorescein angiography; ICGA = indocyanine green angiography. |
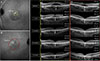 | Fig. 2(A) A case of focal photodynamic therapy (PDT) treatment in a 51-year-old male patient with chronic central subfield thickness (CSC) and (B) a case of conventional PDT treatment in a 58-year-old female patient with chronic CSC. Yellow circle indicates focal PDT area and red circle indicates conventional PDT area in middle-phase indocyanine green angiogram. Note that the focal PDT area was much smaller than the conventional area in the indocyanine green angiography. (C,D) The choroidal thickness (right bottom in optical coherence tomography) and central subfield thickness (right top in optical coherence tomography) were decreased significantly during the follow-up period compared with baseline in both groups. |
References
1. Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994; 14:231–242.
2. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23:288–298.
3. Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34.
4. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992; 81:379–386.
5. Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989; 96:854–859.
6. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22:19–24.
7. Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003; 23:752–763.
8. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005; 139:87–99.
9. Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011; 31:119–126.
10. Lai TY, Chan WM, Li H, et al. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006; 90:869–874.
11. Tsai MJ, Hsieh YT. Half-time photodynamic therapy for central serous chorioretinopathy. Optom Vis Sci. 2014; 91:1140–1145.
12. Kang NH, Kim YT. Change in subfoveal choroidal thickness in central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy. Eye (Lond). 2013; 27:387–391.
13. Smretschnig E, Ansari-Shahrezaei S, Moussa S, et al. Half-fluence photodynamic therapy in acute central serous chorioretinopathy. Retina. 2012; 32:2014–2019.
14. Chan WM, Lai TY, Lai RY, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008; 115:1756–1765.
15. Son BK, Kim K, Kim ES, Yu SY. Long-term outcomes of full-fluence and half-fluence photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmologica. 2019; 241:105–115.
16. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003; 87:1453–1458.
17. Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006; 26:239–242.
18. Chan WM, Lai TY, Lai RY, et al. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008; 28:85–93.
19. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010; 117:1792–1799.
20. Pryds A, Larsen M. Choroidal thickness following extrafoveal photodynamic treatment with verteporfin in patients with central serous chorioretinopathy. Acta Ophthalmol. 2012; 90:738–743.
21. Maruko I, Iida T, Sugano Y, et al. One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina. 2011; 31:1921–1927.
22. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009; 29:1469–1473.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download