Abstract
Cancer immunotherapy has emerged as a promising therapy for a wide variety of tumors. Immune checkpoint inhibitors including anti cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed death 1 (PD-1) and programmed death ligand-1 (PD-L1) monoclonal antibodies have proven to be especially effective in various advanced cancers. However, cancer the immunotherapy disturbs the immune system and may also cause immune related side effects (IRAE) distinguished from cytotoxic chemotherapy toxicity. Among them, endocrine IRAE has been reported with a higher incidence than other organ IRAE. We focus on the most relevant and new aspects related to endocrine IRAE due to cancer immunotherapy in this review.
Figures and Tables
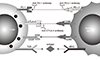 | Fig. 1Mechanism of immune checkpoint inhibitors (Adopted and modified from reference 37). Immune checkpoints are receptors for T lymphocytes which modulate the immune system after binding ligands, either stimulating or inhibiting T cell response. Co-stimulatory immune control points, such as Cluster of Differentiation 28 (CD28), Inducible T-cell COStimulator (ICOS), and Cluster of Differentiation 46 (CD46), are induced by the activated antigen presenting cells (APCs). In contrary, there are two main co-inhibitory immune control points, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death receptor 1 (PD-1). CTLA-4 is expressed on the surface of most activated T lymphocytes during the initial activation phase in lymphatic tissue by dendritic cells and by other APCs. It inhibits periepheral T cell activation, leading to immune tolerance through negative signaling and competitive antagonism of the CD28/B7-mediated co-stimulatory pathway. PD-1 is another co-inhibitory receptor expressed in activated T cell during the effector phase. The binding of PD-1 to its PD-L1 (B7-H1) and PD-L2 (B7-DC) ligands tissue macrophages inhibits T lymphocyte activation facilitating immunological tolerance. Therefore, anti PD-1/PD-L1 and anti CTLA-4 inhibitors facilitate T cell activation and show antitumor effects. |
Table 1
Toxicity grading of endocrine IRAEs of ICIs according to Common Terminology Criteria for Adverse Events (CTCAE) of National Institutes of Health (NIH) (Adopted and modified from reference 38)

aGrade of IRAEs has been estimated according to Common Terminology Criteria for Adverse Events (CTCAE) grading scale; grade 5 relates to death for each IRAE.
ADL: activities of daily living, CCS: corticosteroid, FT4: free thyroxine, ICIs: immune checkpoint inhibitors, MRI: magnetic resonance imaging, NSAIDs: nonsteroidal anti-inflammatory drugs, T1DM: type1 diabetes mellitus, TSH: thyroid stimulating hormone
References
1. Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013; 19(19):5300–5309.

2. Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol (Oxf). 2016; 85(3):331–339.

3. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366(26):2443–2454.

4. Guaraldi F, La Selva R, Sama MT, D'Angelo V, Gori D, Fava P, et al. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. J Endocrinol Invest. 2018; 41(5):549–556.

5. Chalan P, Di Dalmazi G, Pani F, De Remigis A, Corsello A, Caturegli P. Thyroid dysfunctions secondary to cancer immunotherapy. J Endocrinol Invest. 2018; 41(6):625–638.

6. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015; 372(26):2521–2532.

7. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014; 99(11):4078–4085.

8. Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010; 13(1):29–38.

9. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363(8):711–723.

10. Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014; 21(2):371–381.

11. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015; 373(1):23–34.

12. Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017; 86(4):614–620.

13. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016; 101(11):4431–4439.

14. Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015; 100(5):1738–1741.

15. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018; 28(10):1243–1251.

16. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016; 54:139–148.

17. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015; 33(28):3193–3198.

18. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013; 119(9):1675–1682.

19. Bellastella G, Maiorino MI, Bizzarro A, Giugliano D, Esposito K, Bellastella A, et al. Revisitation of autoimmune hypophysitis: knowledge and uncertainties on pathophysiological and clinical aspects. Pituitary. 2016; 19(6):625–642.

20. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013; 98(4):1361–1375.

21. Marlier J, Cocquyt V, Brochez L, Van Belle S, Kruse V. Ipilimumab, not just another anti-cancer therapy: hypophysitis as side effect illustrated by four case-reports. Endocrine. 2014; 47(3):878–883.

22. Iglesias P. Cancer immunotherapy-induced endocrinopathies: clinical behavior and therapeutic approach. Eur J Intern Med. 2018; 47:6–13.

23. Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-alpha production. J Transl Med. 2013; 11:108.

24. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014; 6(230):230ra45.

25. Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013; 1(3):e15.

26. Howard SA, Krajewski KM, Jagannathan JP, Braschi-Amirfarzan M, Tirumani SH, Shinagare AB, et al. A new look at toxicity in the era of precision oncology: imaging findings, their relationship with tumor response, and effect on metastasectomy. AJR Am J Roentgenol. 2016; 207(1):4–14.

27. Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, et al. Anti-PD-1 and Anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015; 38(9):e137–e138.

28. Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015; 38(4):e55–e57.

29. Martin-Liberal J, Furness AJ, Joshi K, Peggs KS, Quezada SA, Larkin J. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015; 64(6):765–767.

30. Gaudy C, Clevy C, Monestier S, Dubois N, Preau Y, Mallet S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015; 38(11):e182–e183.

31. Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016; 4:89.

32. Chae YK, Chiec L, Mohindra N, Gentzler R, Patel J, Giles F. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother. 2017; 66(1):25–32.

33. Win MA, Thein KZ, Qdaisat A, Yeung SJ. Acute symptomatic hypocalcemia from immune checkpoint therapy-induced hypoparathyroidism. Am J Emerg Med. 2017; 35(7):1039.e5–1039.e7.

34. Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019; 145(2):511–521.

35. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019; 145(2):479–485.

36. Abu-Sbeih H, Tang T, Ali FS, Johnson DH, Qiao W, Diab A, et al. The impact of immune checkpoint inhibitor-related adverse events and their immunosuppressive treatment on patients' outcomes. J Immunother Precis Oncol. 2018; 1(1):7–18.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download